Utilization strategies of inorganic nitrogen in submerged macrophytes
-
摘要:
沉水植物在水体生态系统中发挥着关键作用,能通过直接和间接途径缓解水体中的氮负荷,促进湖泊生态系统的良性运转。沉水植物在无机氮的利用策略上与陆生植物差异显著,为了适应水环境,沉水植物地上和地下部分能够同时获取环境中的氮,并将其进行向上或向下的运输。为了减少高浓度氮的毒害,沉水植物地上和地下部分在氮利用中存在一定的权衡关系。沉水植物氮同化的主要场所是叶片,主要通过谷氨酰胺合成酶/谷氨酸合成酶(GS /GOGAT)循环和谷氨酸脱氢酶(GDH)途径进行氮的同化。目前针对沉水植物的相关研究还远不及陆生植物,仅涉及到生理响应层面,仍需深入探索沉水植物氮素利用的机理,开发合适的遗传转化体系,并利用基因编辑等技术对基因功能进行验证,对关键蛋白质的结构与功能展开深入研究。
Abstract:As important components in aquatic ecosystems, submerged macrophytes can alleviate nitrogen load and improve the healthy operation of ecosystems through direct and indirect ways. Nitrogen utilization strategies of submerged macrophytes differ significantly from those of terrestrial plants. Submerged macrophytes can uptake nitrogen not only from the overlying water, but also from pore water in sediments via above-ground and below-ground parts, respectively. To adapt to the various changes in nitrogen content in aquatic environments, submerged macrophytes exhibit two directions of nitrogen translocation, namely acropetal and basipetal translocation. To avoid the toxicity caused by high nitrogen concentrations, a trade-off exists in nitrogen utilization between the above- and below-ground parts of submerged macrophytes. The GS/GOGAT cycle and GDH pathway are the primary pathways for nitrogen assimilation in submerged macrophytes. Currently, research on submerged macrophytes lags far behind that on terrestrial plants. Further exploration of the mechanisms underlying nitrogen utilization in submerged macrophytes is still needed and a suitable genetic transformation system of submerged macrophytes is required. Molecular technologies such as gene editing can be used to identify gene function, which should promote further studies on the structure and function of key proteins.
-
1. 沉水植物及其重要性
沉水植物是水体生态系统中不可或缺的一部分,在关键界面上扮演着重要角色,对湖泊的生产力和关键生源要素产生显著影响[1],对维持生态系统的结构和功能具有重要作用[2, 3]。氮(N)素对植物的生长和繁殖至关重要[4],植物可以从周围环境中吸收大量的氮素,并利用其进行氨基酸、蛋白质、DNA和其他含氮化合物的生物合成[5]。近年来,营养物质对水域生态系统健康的影响受到人们的广泛关注[6-8]。研究表明,高浓度的氮不仅会改变水域生态系统的结构,还会加速水体从清澈的稳定状态转变为浑浊的稳定状态[5, 7, 9, 10],进而对生态系统的功能产生不利影响。氮是植物生长发育所需的矿质元素,沉水植物能够通过多种方式(如直接吸收、通过构建稳定的植物-微生物自净系统等)有效降低水体的氮浓度,改善水体的营养水平,使湖泊生态系统维持稳定的健康状态[11, 12]。
2. 无机氮
氮对植物的生长发育具有重要作用,在植物生命周期中至关重要[13]。陆生植物不仅能够通过根从土壤中吸收氮,其叶片也具有氮吸收能力[14]。但由于大气中的氮主要以N2分子的形式存在,氮原子间的共价键具有较高的键能,很难被破坏[15]。自然界中只有部分微生物(如固氮微生物)和大气放电作用才能将空气中的氮气转化为植物可利用的氮[13, 15]。因此,传统观念认为陆生植物主要通过根部从土壤中吸收氮,氮进入植物细胞后,一部分被运输到地上部进行生物合成,剩余部分直接在根部被合成小分子氨基酸或储存在液泡中[16]。
硝态氮(NO3−-N)是环境中无机氮的主要形态之一,由于其很难与土壤形成表面复合物,因此在环境中容易流失[17]。土壤中的NO3−-N浓度波动较大,为了适应其浓度变化,植物形成了两种转运吸收系统:高亲和力转运系统(HATS)和低亲和力转运系统(LATS)[18]。NO3−-N在两种转运系统的作用下通过主动运输进入植物体,其中一部分会在细胞质中被还原,并同化为氨基酸,而另一部分可储备在细胞的液泡中[19]。根系吸收的氮素可通过木质部在蒸腾作用的拉力下向上运输,然后在地上部分的细胞中通过各种方式被植物同化[20]。
与NO3−-N相比,环境中的铵态氮(NH4+-N)更容易被植物吸收。植物吸收的NO3−-N可在酶促反应下还原为NH4+-N,植物也可以直接从环境中吸收NH4+-N,在此基础上进行同化等生理过程。因此,在植物体中,NH4+-N的同化比NO3−-N更加节能[20, 21]。然而,NH4+-N是一把双刃剑,其在低浓度时能促进植物的正常生理代谢,有效提高植物的生长繁殖能力。但当其浓度过高时,也会对植物产生严重的胁迫作用[22],且NH4+-N对植物的胁迫效应和毒性高于NO3−-N [8, 23, 24]。
3. 沉水植物对无机氮的吸收与运输
对于无机氮的吸收与运输,在陆生植物中的研究已经深入到分子机理层面。研究表明,对于NO3−-N的吸收与运输,目前在拟南芥(Arabidopsis thaliana (L.) Heynh.)中已发现NRT1和NRT2两大基因家族[19, 20, 23]。当环境中的NO3−处于较高浓度时,植物体内氮的吸收及运输主要受NRT1基因家族的主导,通过对LATS的调控,降低对环境中氮的吸收,以避免高氮对植物的抑制作用。研究表明,NRT1.1不仅参与植物对环境中NO3−-N吸收转运的调控,还能调控气孔的开放[21]、种子的休眠[25]、生长素的分泌[26]等代谢过程。与NRT1不同的是,NRT2的表达不受NO3−-N的诱导,当环境中NO3−的浓度较低,植物处于缺氮条件时,NRT2家族主要对HATS进行调控。在拟南芥中,NRT2.1和NRT2.2在HATS中起主要作用[27]。NRT2.4的表达受NO3−-N浓度的调控,对NO3−-N的响应浓度较广。当环境中NO3−-N不足时,植物NRT2.4在根及嫩枝中均能起到NO3−-N的转运作用[28]。
尽管环境中铵态氮的浓度通常低于硝态氮,但植物更偏向于吸收铵态氮 [21, 29]。为了进入植物细胞,植物根部的铵态氮需要依靠细胞膜上的特异性转运蛋白[30]。研究发现,当环境中NH4+-N的浓度低于0.5 mmol/L时,植物主要通过HATS进行铵的吸收和转运;当浓度超过0.5 mmol/L时,植物则改变策略,通过LATS对铵进行吸收及转运[31]。在拟南芥中发现,AMT1.1、AMT1.2、AMT1.3等基因主要负责根部NH4+-N的吸收[32, 33]。其中,AMT1.2在根部皮质层和内皮层中高表达,而AMT1.3主要在表皮层和皮质层中大量表达[32, 34]。AMT1.3的表达与外界NH4+-N的浓度密切相关[35],且受光照强度的影响,因此被认为参与植物C-N平衡的调节[35]。此外,在毛果杨(Populus trichocarpa Torr. & A. Gray ex Hook)和水稻(Oryza sativa L.)的基因组中分别发现14个和10个AMT相关基因[36, 37]。NH4+-N的吸收受多个AMT基因的协同调控,且这种调控受植物自身及其生存环境的影响[4]。沉水植物作为湖泊生态系统中重要的生物类群,不仅是湖泊生态系统多样性的基础,也是其健康运转的关键,在决定水生态系统的健康和功能上具有重要作用[34, 38, 39]。
关于沉水植物对氮的吸收,现有报道多从吸收动力学的角度展开研究。Xiong[40]发现伊乐藻(Elodea nuttallii Michx.)和苦草(Vallisneria natans (Lour.) H. Hara)对氮的吸收与水体中的磷浓度密切相关,随磷浓度的增加,两种沉水植物对NH4+-N和NO3−-N的最大吸收速率均呈下降趋势,但对NH4+-N吸收的Km值均呈上升趋势;两种植物对NO3−-N吸收的Km值趋势相反,在伊乐藻中为下降趋势,而在苦草中则呈上升趋势。Olesen等[41]研究了4种沉水植物(穿叶眼子菜(Potamogeton perfoliatus L.)、钝叶眼子菜(P. obtusifolius Mert. & W. D. J. Koch)、加拿大伊乐藻(E. canadensis Michx.)和小水毛茛(Ranunculus aquatilis L.))对不同氮素的吸收动力学。结果发现,钝叶眼子菜的Vmax最大,穿叶眼子菜和加拿大伊乐藻的较低。对苦草和黑藻(Hydrilla verticillata (L. f.) Royle)铵态氮吸收动力学的研究发现,苦草对铵态氮具有更高的吸收效率[42]。研究表明,吸收效率与外界水环境浓度密切相关,当NH4+-N的浓度为3 mg/L左右时,圆叶节节菜(Rotala rotundifolia (Buch.-Ham. ex Roxb.) Koehne)对其具有最高的吸收效率[43]。然而,也有研究指出,沉水植物对铵态氮的吸收速率随外界NH4+-N浓度的升高呈先上升后下降的趋势[44]。
目前,对沉水植物氮吸收的研究主要集中在吸收动力学,少量报道开始利用转录组分析从分子水平对其调控机理开展探讨。沉水植物通过铵转运蛋白家族(AMTs)实现水体铵态氮的吸收和转运,在NH4+-N浓度较低的环境条件下,粉绿狐尾藻(Myriophyllum aquaticum (Vell.) Verdc.)编码铵转运蛋白的基因AMT1.2和AMT3.1的表达显著上调,而当环境中NH4+-N的浓度增加时,基因的表达显著下调[45]。
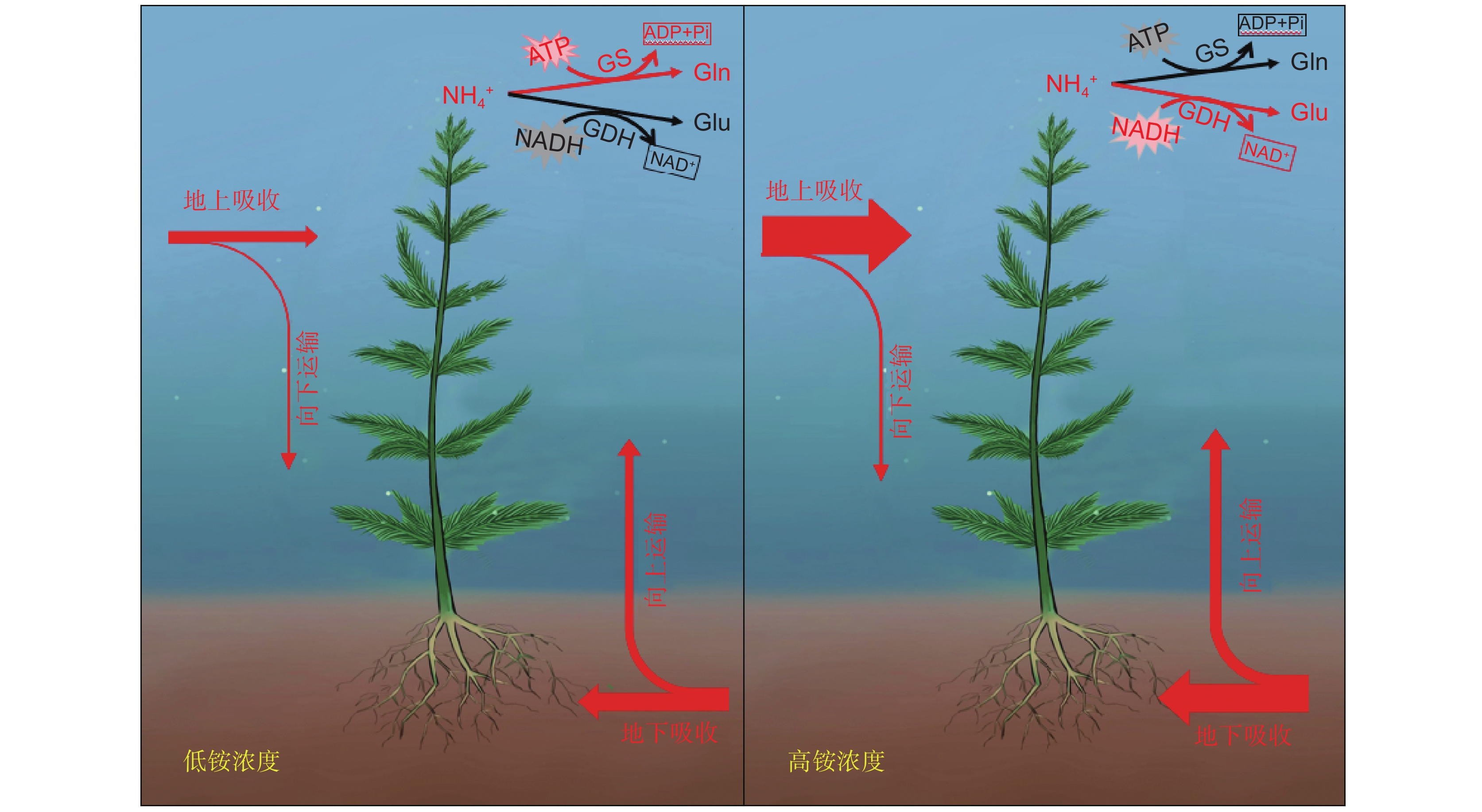
沉水植物既可以通过根部从环境中吸收氮,也可以通过叶片等部位直接吸收水体中游离的氮。而陆生植物所需要的氮源主要通过根部的吸收、转运和同化[14],叶片仅能通过固氮菌实现极少量的氮固定,叶片中的氮主要来自根的向上运输过程。因此,沉水植物与陆生植物的叶片在氮的利用上可能存在显著差异,且氮在沉水植物体内的运输也可能与陆生植物不同[46]。陆生植物通常以自下而上的单向运输为主,当根部接收到高浓度氮源时,可以通过低亲和力转运系统减少根部对外源氮的吸收,或者将根部吸收的氮转移至地上部分,以降低高浓度氮对植物根系的毒害作用。当处于氮限制环境条件时,可以通过高亲和力转运系统增加根部氮的吸收,并向上运输,以满足地上部分的氮需求[47, 48]。沉水植物由于地下和地上部分均能获取环境中的氮[49-51],为了适应水环境中氮含量的变化,其对氮的运输存在向上及向下的双向运输。为了减轻高浓度氮对自身的毒害,沉水植物对氮的利用在地上部分(叶等器官)和地下部分(根或根茎)存在一定的权衡关系。我们近期的研究表明,沉水植物对外源铵的转运存在明显的双向运输(图1)。当环境中的氮含量受限时,沉水植物主要通过地下部分从沉积物中吸收氮,并向上运输至地上部分;而当环境中的铵浓度过高时,沉水植物的双向运输依然存在,其地上部分能够通过迅速的氮合成显著降低植物体中游离氮的积累,从而避免毒害作用的产生[42]。
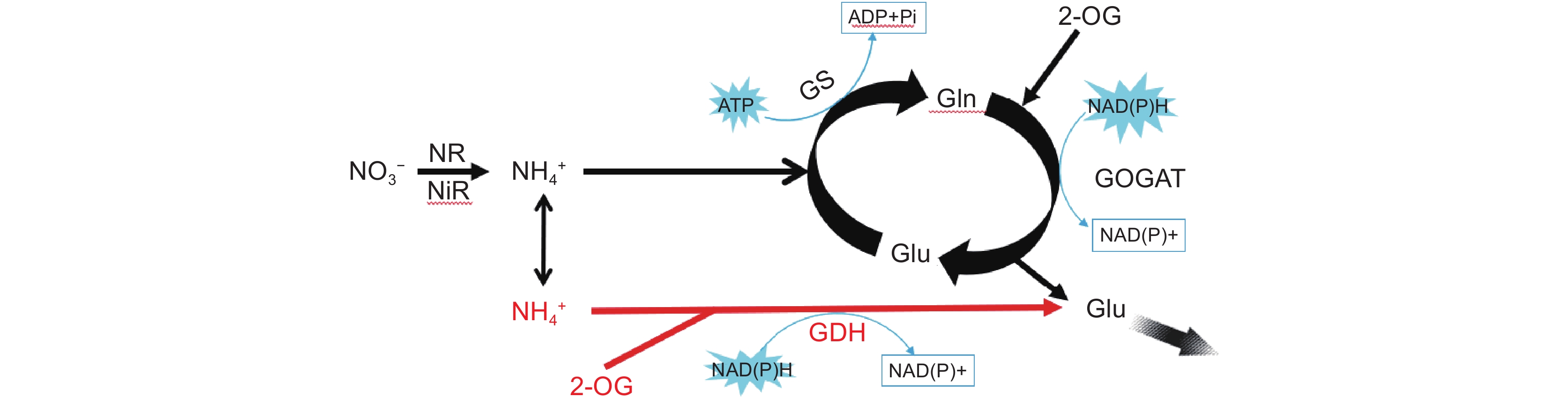
![]() 图 1 沉水植物在不同铵氮浓度条件下的吸收及运输过程(改自Xian等[46])箭头宽度代表植物地上及地下部分对外源铵态氮吸收及转运相对量的大小。GS:谷氨酰胺合成酶,GDH:谷氨酸脱氢酶,Glu:谷氨酸,Gln:谷氨酰胺。红色代谢途径为当前环境条件下植物铵同化的主要途径。Figure 1. Ammonium uptake and transportation in submerged plants under different ammonium concentrations (modified from Xian et al.[46])Arrow width represents relative amount of external ammonium uptake and transport by aboveground and underground parts of submerged macrophytes. GS: glutamine synthetase, GDH: glutamate dehydrogenase, Glu: glutamate, Gln: glutamine. Red metabolic pathways indicate primary route for ammonium assimilation under current environmental conditions.
图 1 沉水植物在不同铵氮浓度条件下的吸收及运输过程(改自Xian等[46])箭头宽度代表植物地上及地下部分对外源铵态氮吸收及转运相对量的大小。GS:谷氨酰胺合成酶,GDH:谷氨酸脱氢酶,Glu:谷氨酸,Gln:谷氨酰胺。红色代谢途径为当前环境条件下植物铵同化的主要途径。Figure 1. Ammonium uptake and transportation in submerged plants under different ammonium concentrations (modified from Xian et al.[46])Arrow width represents relative amount of external ammonium uptake and transport by aboveground and underground parts of submerged macrophytes. GS: glutamine synthetase, GDH: glutamate dehydrogenase, Glu: glutamate, Gln: glutamine. Red metabolic pathways indicate primary route for ammonium assimilation under current environmental conditions.4. 沉水植物对无机氮的同化
植物氮同化是合成含氮有机化合物的关键步骤,一般而言,该步骤涉及多种酶的参与,包括硝酸还原酶(NR)、亚硝酸还原酶(NiR)、谷氨酰胺合成酶(GS)、谷氨酸合成酶(GOGAT)和谷氨酸脱氢酶(GDH)等。植物吸收硝态氮后,一部分储存在木质部,或者芽和根等器官的液泡中;另一部分在细胞质中被NR还原为NO2–,后者被植物细胞迅速运输至质体中还原,最终以NH4+的形式被植物同化[33, 52]。植物体内的NH4+-N主要通过GS-GOGAT(GS/GOGAT循环途径)被同化(图2)。其中,GS对NH4+有较高的亲和力,作为氮代谢过程的关键酶,能够将铵转化为谷氨酰胺,后者在GOGAT的作用下结合一分子的谷氨酸,与α-酮戊二酸(2-OG)结合,产生两分子的谷氨酸[53-55]。植物体中的GOGAT有两种亚型:Fd-GOGAT和NADH-GOGAT,前者主要从还原性铁中获取电子[56],而后者则主要依赖呼吸作用产生的NADH提供电子[57]。除了GS-GOGAT循环, GDH催化的代谢途径也被认为是植物铵同化的一条途径。GDH在植物体内广泛存在,是一种分子量为208~300 kDa的蛋白质[58],通常形成六聚体[59]。它能催化NH4+和α-酮戊二酸结合生成谷氨酸,同时也能催化谷氨酸的分解(图2)。在高等植物中,GDH分为两种类型:以NADPH为电子供体的NADPH-GDH存在于叶绿体中,而以NADH为电子供体的NADH-GDH则存在于线粒体中[59]。尽管早期研究普遍认为GDH催化的代谢途径是植物进行铵同化的主要途径,但到了1976年,由于GOGAT的发现,GS-GOGAT循环才被认为是高等植物同化铵的主要途径[60]。目前,通过对GS-GOGAT循环和GDH途径的深入探讨,研究者更深入地比较了植物中铵同化途经的特征。如,在铵胁迫条件下,黄瓜(Cucumis sativus L.)叶片主要通过GS-GOGAT循环途经同化铵,而根部则主要通过GDH途经来同化[61]
![]() 图 2 植物无机氮同化路径GS:谷氨酰胺合成酶;GDH:谷氨酸脱氢酶;GOGAT:谷氨酸合成酶;Glu:谷氨酸;Gln:谷氨酰胺;2-OG:α-酮戊二酸。红色和黑色分别代表两条无机氮同化途径:黑色为GS/GOGAT循环途径,红色为GDH途径。Figure 2. Inorganic nitrogen assimilation pathways in plantsGS: Glutamine synthetase; GDH: Glutamate dehydrogenase; GOGAT: Glutamate synthase; Glu: Glutamate; Gln: Glutamine; 2-OG: α-ketoglutarate. Red and black represent two pathways to inorganic nitrogen assimilation: black denotes GS/GOGAT cycle pathway, red denotes GDH pathway.
图 2 植物无机氮同化路径GS:谷氨酰胺合成酶;GDH:谷氨酸脱氢酶;GOGAT:谷氨酸合成酶;Glu:谷氨酸;Gln:谷氨酰胺;2-OG:α-酮戊二酸。红色和黑色分别代表两条无机氮同化途径:黑色为GS/GOGAT循环途径,红色为GDH途径。Figure 2. Inorganic nitrogen assimilation pathways in plantsGS: Glutamine synthetase; GDH: Glutamate dehydrogenase; GOGAT: Glutamate synthase; Glu: Glutamate; Gln: Glutamine; 2-OG: α-ketoglutarate. Red and black represent two pathways to inorganic nitrogen assimilation: black denotes GS/GOGAT cycle pathway, red denotes GDH pathway.对于沉水植物而言,由于水体中的无机氮主要以NO3−-N和NH4+-N的形式存在,植物更倾向于利用NH4+-N,因为消耗的能量更少[62, 63]。沉水植物的氮同化过程与陆生植物类似,但在同化部位、同化路径的选择等方面有较大差异。由于直接受水体氮浓度波动的影响,沉水植物主要通过叶片进行氮素的同化和利用[42]。从环境中吸收氮后,沉水植物通常会将其合成含氮有机物储存在体内,以避免氮在体内积累导致的毒害[64, 65]。目前,相对于陆生植物,对沉水植物氮同化的机制研究还不够深入,其氮同化途径的特征仍不明确。仅有少量报道比较了胁迫条件下水生植物铵同化过程中关键酶的活性。譬如,水体中NH4+-N的浓度和pH值显著影响金鱼藻(Ceratophyllum demersum L.)GS的活性,在pH值为9的溶液中,GS活性显著下降;同样,在高浓度NH4+-N条件下,GS活性随着胁迫时间的增加也呈显著下降趋势[66]。粉绿狐尾藻也表现出类似的趋势,当环境中NH4+-N浓度过高时,其叶片中GS的活性被显著抑制,但当NH4+-N浓度为120 mg/L时,叶片中NR的活性迅速上升[67]。转录组分析发现,随着水体NH4+-N浓度的升高,粉绿狐尾藻叶片中编码GS的关键基因的表达量显著下降,而多个GDH和GOGAT的编码基因的表达则显著上调[61]。
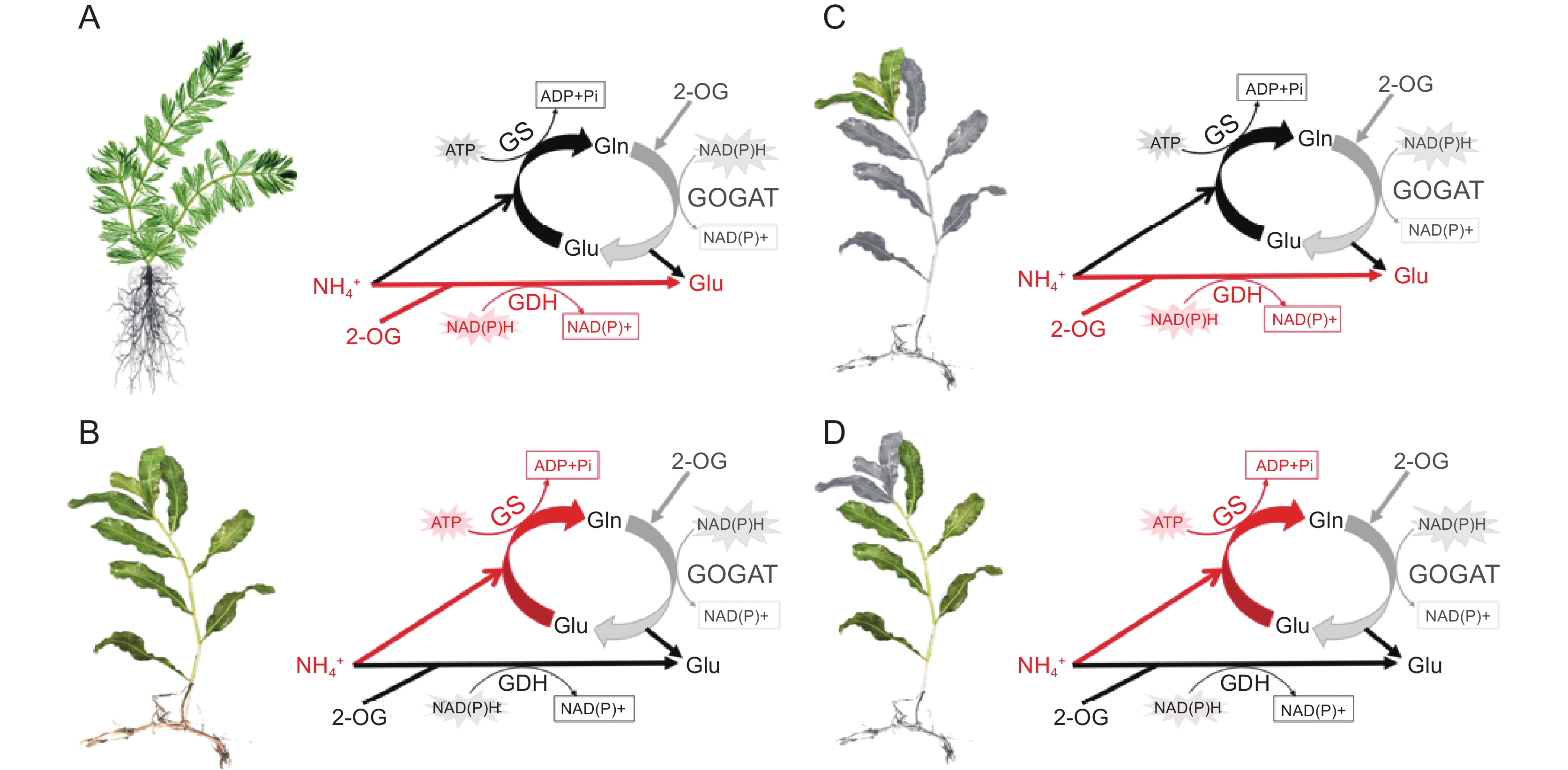
最近研究发现,沉水植物对铵的同化特征受物种及生长时期的影响。在高浓度铵态氮条件下,铵耐受型物种穗花狐尾藻(M. spicatum L.)主要通过GDH途径进行铵的同化作用[46, 68],而铵敏感型物种光叶眼子菜(P. lucen L.)主要通过GS-GOGAT途径对铵进行同化(图3:A、B)[69]。针对同一物种不同生长时期的研究发现,沉水植物幼叶主要通过GDH途径同化水体中的铵态氮,而成熟叶在正常浓度条件下主要通过GS-GOGAT途径实现铵态氮的同化(图3:C、D)[69]。此外,比较转录组分析结果进一步表明,沉水植物光叶眼子菜谷氨酸脱氢酶基因GDH2在不同浓度铵态氮条件下的表达量存在显著差异。
![]() A:高浓度铵态氮条件下,耐受型物种穗花狐尾藻铵同化以GDH途径为主导;B:敏感型物种光叶眼子菜铵同化以GS/GOGAT途径为主导; C:光叶眼子菜幼叶主要通过GDH途径进行铵的同化;D:成熟叶主要通过GS/GOGAT循环进行铵的同化。缩写同图2。Figure 3. Inorganic nitrogen assimilation pathways in submerged macrophytes with different adaptive capacities and developmental stages (modified from Xian et al.[46, 68] and Ochieng et al.[69])A: Ammonium assimilation in ammonium-tolerant species M. spicatum is dominated by GDH pathway under high ammonium concentration. B: Ammonium assimilation in ammonium-sensitive species P. lucens is dominated by GS/GOGAT pathway. C: In young leaves of P. lucens, ammonium assimilation is primarily mediated through the GDH pathway. D: In mature leaves of P. lucens, ammonium assimilation is mainly mediated through the GS/GOGAT cycle. Abbreviations are as in Fig. 2.
A:高浓度铵态氮条件下,耐受型物种穗花狐尾藻铵同化以GDH途径为主导;B:敏感型物种光叶眼子菜铵同化以GS/GOGAT途径为主导; C:光叶眼子菜幼叶主要通过GDH途径进行铵的同化;D:成熟叶主要通过GS/GOGAT循环进行铵的同化。缩写同图2。Figure 3. Inorganic nitrogen assimilation pathways in submerged macrophytes with different adaptive capacities and developmental stages (modified from Xian et al.[46, 68] and Ochieng et al.[69])A: Ammonium assimilation in ammonium-tolerant species M. spicatum is dominated by GDH pathway under high ammonium concentration. B: Ammonium assimilation in ammonium-sensitive species P. lucens is dominated by GS/GOGAT pathway. C: In young leaves of P. lucens, ammonium assimilation is primarily mediated through the GDH pathway. D: In mature leaves of P. lucens, ammonium assimilation is mainly mediated through the GS/GOGAT cycle. Abbreviations are as in Fig. 2.5. 展望
近年来,尽管沉水植物氮的吸收与利用受到了广泛关注,但仍需在不同物种(广度)及不同研究层次(深度)上开展细致的研究。
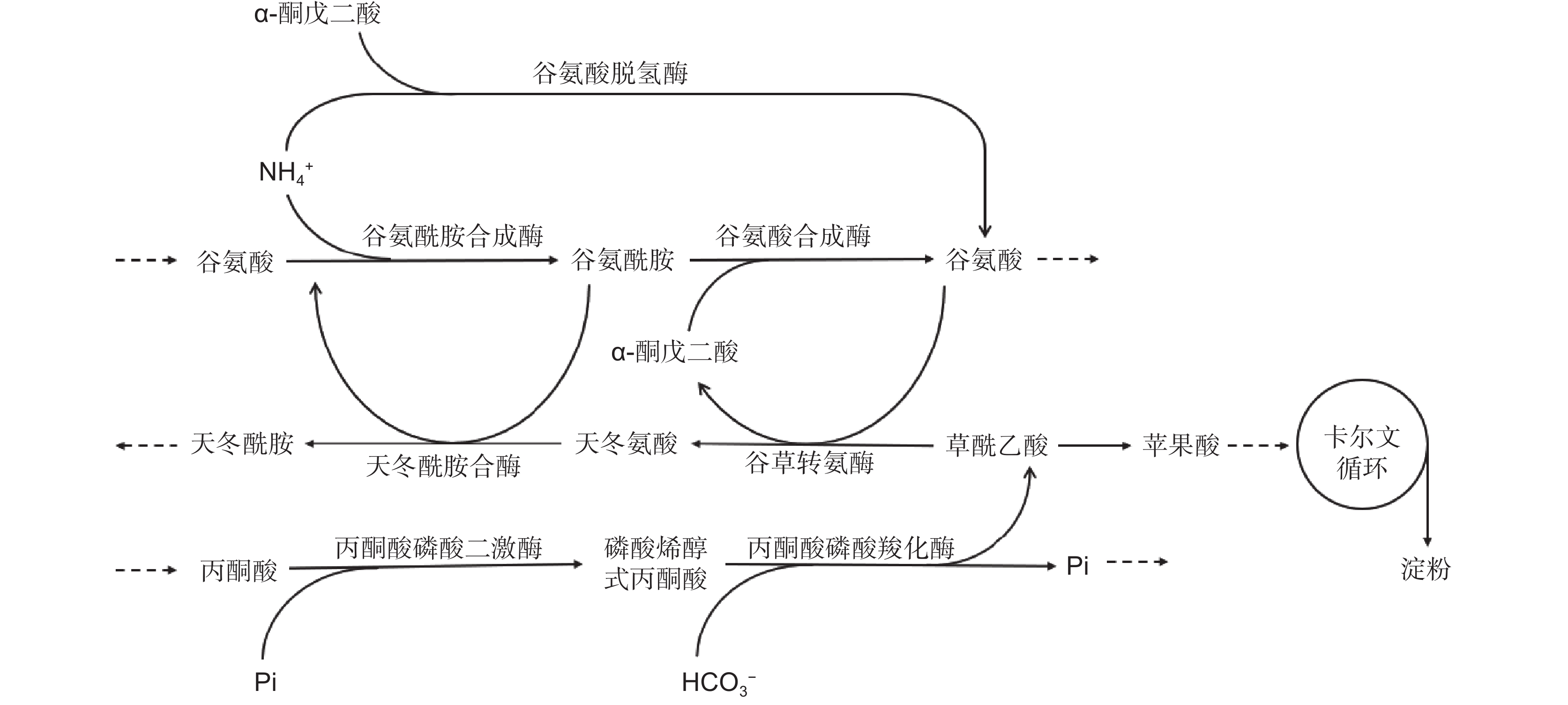
沉水植物的叶片能够利用水体的无机氮,并在细胞内通过一系列酶促反应最终形成谷氨酰胺和谷氨酸。我们近期通过代谢通路关联性分析发现,产生的谷氨酸能够在植物体内参与多种代谢过程,进而被植物高效利用,在众多代谢通路中,关系最紧密的是植物的光合碳同化(图4):(1)沉水植物铵同化过程中产生的谷氨酰胺能与α-酮戊二酸结合,在谷氨酸合成酶的催化作用下生成谷氨酸;(2)谷氨酸脱氢酶能够直接催化2-OG与铵结合,并生成谷氨酸;(3)谷氨酸和草酰乙酸(OAA)能够在谷草转氨酶(GOT)的作用下生成天冬氨酸和α-酮戊二酸。在此过程中,沉水植物主要通过α-酮戊二酸和草酰乙酸将碳氮代谢进行关联。然而,在不同氮素浓度条件下,沉水植物通过何种途径实现2-OG 和OAA在碳氮联合利用过程中的调控还不清楚,相关机制还需要深入研究和发掘。
尽管对沉水植物氮的吸收、运输和同化过程等方面开展了相关探讨,但研究深度还远远不够。目前仅在生理响应层面,通过酶活性的变化,揭示了无机氮同化中的关键代谢途径[68, 69],但对这些关键酶的活性及其基因表达的调控方式还缺乏相应研究,目前仅有少量报道从转录组层面进行了分析[70]。研究难以深入的原因主要是沉水植物尚未构建成熟的遗传转化系统,且缺乏模式植物。此外,尽管沉水植物的基因组总体较小,但仅有少量物种的全基因组序列被发表[20]。因此,还需进一步探索研究方法,构建合适的遗传转化体系,同时采取最新的基因编辑等分子技术,从而实现对沉水植物功能基因的验证,以及对关键蛋白质结构和功能的深入研究。
-
图 1 沉水植物在不同铵氮浓度条件下的吸收及运输过程(改自Xian等[46])
箭头宽度代表植物地上及地下部分对外源铵态氮吸收及转运相对量的大小。GS:谷氨酰胺合成酶,GDH:谷氨酸脱氢酶,Glu:谷氨酸,Gln:谷氨酰胺。红色代谢途径为当前环境条件下植物铵同化的主要途径。
Figure 1. Ammonium uptake and transportation in submerged plants under different ammonium concentrations (modified from Xian et al.[46])
Arrow width represents relative amount of external ammonium uptake and transport by aboveground and underground parts of submerged macrophytes. GS: glutamine synthetase, GDH: glutamate dehydrogenase, Glu: glutamate, Gln: glutamine. Red metabolic pathways indicate primary route for ammonium assimilation under current environmental conditions.
图 2 植物无机氮同化路径
GS:谷氨酰胺合成酶;GDH:谷氨酸脱氢酶;GOGAT:谷氨酸合成酶;Glu:谷氨酸;Gln:谷氨酰胺;2-OG:α-酮戊二酸。红色和黑色分别代表两条无机氮同化途径:黑色为GS/GOGAT循环途径,红色为GDH途径。
Figure 2. Inorganic nitrogen assimilation pathways in plants
GS: Glutamine synthetase; GDH: Glutamate dehydrogenase; GOGAT: Glutamate synthase; Glu: Glutamate; Gln: Glutamine; 2-OG: α-ketoglutarate. Red and black represent two pathways to inorganic nitrogen assimilation: black denotes GS/GOGAT cycle pathway, red denotes GDH pathway.
图 3 不同适应能力及不同发育时期沉水植物的无机氮同化路径(改自Xian等[46, 68]和Ochieng等[69])
A:高浓度铵态氮条件下,耐受型物种穗花狐尾藻铵同化以GDH途径为主导;B:敏感型物种光叶眼子菜铵同化以GS/GOGAT途径为主导; C:光叶眼子菜幼叶主要通过GDH途径进行铵的同化;D:成熟叶主要通过GS/GOGAT循环进行铵的同化。缩写同图2。
Figure 3. Inorganic nitrogen assimilation pathways in submerged macrophytes with different adaptive capacities and developmental stages (modified from Xian et al.[46, 68] and Ochieng et al.[69])
A: Ammonium assimilation in ammonium-tolerant species M. spicatum is dominated by GDH pathway under high ammonium concentration. B: Ammonium assimilation in ammonium-sensitive species P. lucens is dominated by GS/GOGAT pathway. C: In young leaves of P. lucens, ammonium assimilation is primarily mediated through the GDH pathway. D: In mature leaves of P. lucens, ammonium assimilation is mainly mediated through the GS/GOGAT cycle. Abbreviations are as in Fig. 2.
-
[1] Philbrick CT,Les DH. The evolution of aquatic plants:an introduction and tribute to Cyril Duncan Sculthorpe,author of the biology of aquatic vascular plants[J]. Aquat Bot,1993,44(2-3):101−104. doi: 10.1016/0304-3770(93)90067-7
[2] Carpenter SR,Lodge DM. Effects of submersed macrophytes on ecosystem processes[J]. Aquat Bot,1986,26:341−370. doi: 10.1016/0304-3770(86)90031-8
[3] 严雪,于丹,李永科. CO2浓度升高对沉水克隆植物生长速率及营养元素积累的影响[J]. 植物生态学报,2003,27(4):435−440. doi: 10.3321/j.issn:1005-264X.2003.04.001 Yan X,Yu D,Li YK. Response of growth rate and nutrient elements accumulation in submerged clonal plants macrophyte under elevated CO2[J]. Acta Phytoecologica Sinica,2003,27(4):435−440. doi: 10.3321/j.issn:1005-264X.2003.04.001
[4] Kiba T,Krapp A. Plant nitrogen acquisition under low availability:regulation of uptake and root architecture[J]. Plant Cell Physiol,2016,57(4):707−714. doi: 10.1093/pcp/pcw052
[5] Kusano M,Fukushima A,Redestig H,Saito K. Metabolomic approaches toward understanding nitrogen metabolism in plants[J]. J Exp Bot,2011,62(4):1439−1453. doi: 10.1093/jxb/erq417
[6] Özkan K,Jeppesen E,Johansson LS,Beklioglu M. The response of periphyton and submerged macrophytes to nitrogen and phosphorus loading in shallow warm lakes:a mesocosm experiment[J]. Freshwater Biol,2010,55(2):463−475. doi: 10.1111/j.1365-2427.2009.02297.x
[7] Toniciolli Rigueto CV,Rosseto M,Loss RA,dos Santos Richards NSP,Dettmer A,Pizzutti IR. Gelatin-based polymeric films for applications in food packaging:an overview of advances,challenges,and perspectives[J]. Ciênc Rural,2023,53(2):e20210679.
[8] Siefert A,Violle C,Chalmandrier L,Albert CH,Taudiere A,et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities[J]. Ecol Lett,2015,18(12):1406−1419. doi: 10.1111/ele.12508
[9] Barker T,Hatton KO,Connor L,Moss B. Effects of nitrate load on submerged plant biomass and species richness:results of a mesocosm experiment[J]. Fundam Appl Limnol,2008,173(2):89−100. doi: 10.1127/1863-9135/2008/0173-0089
[10] Pan YD,Birdsey RA,Fang JY,Houghton R,Kauppi PE,et al. A large and persistent carbon sink in the world’s forests[J]. Science,2011,333(6045):988−993. doi: 10.1126/science.1201609
[11] Olsen S,Chan F,Li W,Zhao ST,Søndergaard M,Jeppesen E. Strong impact of nitrogen loading on submerged macrophytes and algae:a long-term mesocosm experiment in a shallow Chinese lake[J]. Freshwater Biol,2015,60(8):1525−1536. doi: 10.1111/fwb.12585
[12] 常诏峰. 高原沉水植物对铵盐的生理响应研究[D]. 拉萨:西藏大学,2021:52−55. [13] Mokhele B,Zhan XJ,Yang GZ,Zhang XL. Review:nitrogen assimilation in crop plants and its affecting factors[J]. Can J Plant Sci,2012,92(3):399−405. doi: 10.4141/cjps2011-135
[14] Peuke AD,Jeschke WD,Hartung W. Foliar application of nitrate or ammonium as sole nitrogen supply in Ricinus communis. Ⅱ. The flows of cations,chloride and abscisic acid[J]. New Phytol,1998,140(4):625−636. doi: 10.1046/j.1469-8137.1998.00304.x
[15] Rees DC,Howard JB. Nitrogenase:standing at the crossroads[J]. Curr Opin Chem Biol,2000,4(5):559−566. doi: 10.1016/S1367-5931(00)00132-0
[16] Li FL,Bao WK. New insights into leaf and fine-root trait relationships:implications of resource acquisition among 23 xerophytic woody species[J]. Ecol Evol,2015,5(22):5344−5351. doi: 10.1002/ece3.1794
[17] Strahm BD,Harrison RB. Nitrate sorption in a variable-charge forest soil of the Pacific northwest[J]. Soil Sci,2006,171(4):313−321. doi: 10.1097/01.ss.0000209355.76407.16
[18] 黄慧彧. 根系修剪对水稻幼苗根系形态特征和氮素吸收的影响[D]. 武汉:华中农业大学,2022:10−21. [19] Crawford NM,Glass ADM. Molecular and physiological aspects of nitrate uptake in plants[J]. Trends Plant Sci,1998,3(10):389−395. doi: 10.1016/S1360-1385(98)01311-9
[20] Kattge J,Bönisch G,Díaz S,Lavorel S,Prentice IC,et al. TRY plant trait database-enhanced coverage and open access[J]. Global Change Biol,2020,26(1):119−188. doi: 10.1111/gcb.14904
[21] Sun ZZ,Liu LL,Ma YC,Yin GD,Zhao C,et al. The effect of nitrogen addition on soil respiration from a nitrogen-limited forest soil[J]. Agric For Meteorol,2014,197:103−110. doi: 10.1016/j.agrformet.2014.06.010
[22] Jampeetong A,Brix H. Effects of NH4+ concentration on growth,morphology and NH4+ uptake kinetics of Salvinia natans[J]. Ecol Eng,2009,35(5):695−702. doi: 10.1016/j.ecoleng.2008.11.006
[23] Aslam M,Travis RL,Huffaker RC. Comparative kinetics and reciprocal inhibition of nitrate and nitrite uptake in roots of uninduced and induced barley (Hordeum vulgare L.) seedlings[J]. Plant Physiol,1992,99(3):1124−1133. doi: 10.1104/pp.99.3.1124
[24] 鲜玲. 沉水植物铵同化特征的研究——以穗状狐尾藻和光叶眼子菜为例[D]. 北京:中国科学院大学,2020:52−59. [25] Alboresi A,Gestin C,Leydecker MT,Bedu M,Meyer C,Truong HN. Nitrate,a signal relieving seed dormancy in Arabidopsis[J]. Plant Cell Environ,2005,28(4):500−512. doi: 10.1111/j.1365-3040.2005.01292.x
[26] Krouk G,Lacombe B,Bielach A,Perrine-Walker F,Malinska K,et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants[J]. Dev Cell,2010,18(6):927−937. doi: 10.1016/j.devcel.2010.05.008
[27] Filleur S,Dorbe MF,Cerezo M,Orsel M,Granier F,et al. An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake[J]. FEBS Lett,2001,489(2-3):220−224. doi: 10.1016/S0014-5793(01)02096-8
[28] Kiba T,Feria-Bourrellier AB,Lafouge F,Lezhneva L,Boutet-Mercey S,et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants[J]. Plant Cell,2012,24(1):245−258. doi: 10.1105/tpc.111.092221
[29] Nayar S,Loo MGK,Tanner JE,Longmore AR,Jenkins GP. Nitrogen acquisition and resource allocation strategies in temperate seagrass Zostera nigricaulis:uptake,assimilation,and translocation processes[J]. Sci Rep,2018,8(1):17151. doi: 10.1038/s41598-018-35549-3
[30] Ludewig U,Neuhäuser B,Dynowski M. Molecular mechanisms of ammonium transport and accumulation in plants[J]. FEBS Lett,2007,581(12):2301−2308. doi: 10.1016/j.febslet.2007.03.034
[31] Marschner H. Preface to First Edition[M]. Amsterdam:Elsevier,2012:vii.
[32] Yuan LX,Loque D,Kojima S,Rauch S,Ishiyama K,et al. The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters[J]. Plant Cell,2007,19(8):2636−2652. doi: 10.1105/tpc.107.052134
[33] Wang M,Shen QR,Xu GH,Guo SW. New insight into the strategy for nitrogen metabolism in plant cells[J]. Int Rev Cell Mol Biol,2014,310:1−37.
[34] Loqué D,Yuan LX,Kojima S,Gojon A,Wirth J,et al. Additive contribution of AMT1;1 and AMT1;3 to high-affinity ammonium uptake across the plasma membrane of nitrogen-deficient Arabidopsis roots[J]. Plant J,2006,48(4):522−534. doi: 10.1111/j.1365-313X.2006.02887.x
[35] Gazzarrini S,Lejay L,Gojon A,Ninnemann O,Frommer WB,von Wirén N. Three functional transporters for constitutive,diurnally regulated,and starvation-induced uptake of ammonium into Arabidopsis roots[J]. Plant Cell,1999,11(6):937−947.
[36] Couturier J,Montanini B,Martin F,Brun A,Blaudez D,Chalot M. The expanded family of ammonium transporters in the perennial poplar plant[J]. New Phytol,2007,174(1):137−150. doi: 10.1111/j.1469-8137.2007.01992.x
[37] Sonoda Y,Ikeda A,Saiki S,Yamaya T,Yamaguchi J. Feedback regulation of the ammonium transporter gene family AMT1 by glutamine in rice[J]. Plant Cell Physiol,2004,45(12):1396−1402.
[38] Van den Berg MS,Scheffer M,Coops H,Simons J. The role of characean algae in the management of eutrophic shallow lakes[J]. J Phycol,1998,34(5):750−756. doi: 10.1046/j.1529-8817.1998.340750.x
[39] Jeppesen E,Jensen JP,Søndergaard M,Lauridsen T,Pedersen LJ,Jensen L. Top-down control in freshwater lakes:the role of nutrient state,submerged macrophytes and water depth[J]. Hydrobiologia,1997,342:151−164.
[40] Xiong HF. Uptake kinetics of NH4+,NO3− and H2PO4− by submerged macrophytes Elodea nuttallii and Vallisneria natans (JUSSIEU,1826)[J]. Appl Ecol Environ Res,2019,17(1):1027−1037. doi: 10.15666/aeer/1701_10271037
[41] Olesen A,Jensen SM,Alnoee AB,Baattrup-Pedersen A,Lauridsen TL,et al. Nutrient kinetics in submerged plant beds:a mesocosm study simulating constructed drainage wetlands[J]. Ecol Eng,2018,122:263−270. doi: 10.1016/j.ecoleng.2018.08.012
[42] 陈少毅,许超,姚瑶,黄立章,张云涛. 黑藻和苦草对氨氮、硝态氮和磷吸收动力学研究[J]. 环境科学与技术,2012,35(8):34−36. doi: 10.3969/j.issn.1003-6504.2012.08.008 Chen SY,Xu C,Yao Y,Huang LZ,Zhang YT. Uptake kinetics of ammonia,nitrate and phosphorus by submerged macrophytes Hydrilla verticillata and Vallisneria natans[J]. Environmental Science & Technology,2012,35(8):34−36. doi: 10.3969/j.issn.1003-6504.2012.08.008
[43] Gu SY,Hsieh CT,Gandomi YA,Chang JK,Li J,et al. Microwave growth and tunable photoluminescence of nitrogen-doped graphene and carbon nitride quantum dots[J]. J Mater Chem C,2019,7(18):5468−5476. doi: 10.1039/C9TC00233B
[44] 钟爱文,曹特,张萌,倪乐意,谢平. 光照和黑暗条件下苦草(Vallisneria natans)和穗花狐尾藻(Myriophyllum spicatum)对铵态氮的吸收[J]. 湖泊科学,2013,25(2):289−294. doi: 10.3969/j.issn.1003-5427.2013.02.017 Zhong AW,Cao T,Zhang M,Ni LY,Xie P. Uptake of ammonium by Vallisneria natans and Myriophyllum spicatum under light and dark regimes[J]. Journal of Lake Sciences,2013,25(2):289−294. doi: 10.3969/j.issn.1003-5427.2013.02.017
[45] Wang Q,Zhang ZH,Du R,Wang SP,Duan JC,et al. Richness of plant communities plays a larger role than climate in determining responses of species richness to climate change[J]. J Ecol,2019,107(4):1944−1955. doi: 10.1111/1365-2745.13148
[46] Xian L,Ochieng WA,Muthui SW,Otieno DO,Yu SW,et al. The above-ground part of submerged macrophytes plays an important role in ammonium utilization[J]. Front Plant Sci,2022,13:865578. doi: 10.3389/fpls.2022.865578
[47] Tercé-Laforgue T,Dubois F,Ferrario-Méry S,de Crecenzo MAP,Sangwan R,et al. Glutamate dehydrogenase of tobacco is mainly induced in the cytosol of phloem companion cells when ammonia is provided either externally or released during photorespiration[J]. Plant Physiol,2004,136(4):4308−4317. doi: 10.1104/pp.104.047548
[48] Gibson SI. Control of plant development and gene expression by sugar signaling[J]. Curr Opin Plant Biol,2005,8(1):93−102. doi: 10.1016/j.pbi.2004.11.003
[49] Sand-Jensen K,Prahl C. Oxygen exchange with the lacunae and across leaves and roots of the submerged vascular macrophyte,Lobelia dortmanna L.[J]. New Phytol,1982,91(1):103−120. doi: 10.1111/j.1469-8137.1982.tb03296.x
[50] Cedergreen N,Madsen TV. Nitrate reductase activity in roots and shoots of aquatic macrophytes[J]. Aquat Bot,2003,76(3):203−212. doi: 10.1016/S0304-3770(03)00050-0
[51] Cao T,Ni LY,Xie P,Xu J,Zhang M. Effects of moderate ammonium enrichment on three submersed macrophytes under contrasting light availability[J]. Freshwater Biol,2011,56(8):1620−1629. doi: 10.1111/j.1365-2427.2011.02601.x
[52] Paola Rosales E,Florencia Iannone M,Daniela Groppa M,Patricia Benavides M. Nitric oxide inhibits nitrate reductase activity in wheat leaves[J]. Plant Physiol Biochem,2011,49(2):124−130. doi: 10.1016/j.plaphy.2010.10.009
[53] Diaz C,Lemaitre T,Christ A,Azzopardi M,Kato Y,et al. Nitrogen recycling and remobilization are differentially controlled by leaf senescence and development stage in Arabidopsis under low nitrogen nutrition[J]. Plant Physiol,2008,147(3):1437−1449. doi: 10.1104/pp.108.119040
[54] Masclaux-Daubresse C,Reisdorf-Cren M,Orsel M. Leaf nitrogen remobilisation for plant development and grain filling[J]. Plant Biol,2008,10(s1):23−36. doi: 10.1111/j.1438-8677.2008.00097.x
[55] Linka M,Weber APM. Shuffling ammonia between mitochondria and plastids during photorespiration[J]. Trends Plant Sci,2005,10(10):461−465. doi: 10.1016/j.tplants.2005.08.002
[56] Masclaux-Daubresse C,Reisdorf-Cren M,Pageau K,Lelandais M,Grandjean O,et al. Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco[J]. Plant Physiol,2006,140(2):444−456. doi: 10.1104/pp.105.071910
[57] Blanco L,Reddy PM,Silvente S,Bucciarelli B,Khandual S,et al. Molecular cloning,characterization and regulation of two different NADH-glutamate synthase cDNAs in bean nodules[J]. Plant Cell Environ,2008,31(4):454−472. doi: 10.1111/j.1365-3040.2008.01774.x
[58] Turano FJ,Dashner R,Upadhyaya A,Caldwell CR. Purification of mitochondrial glutamate dehydrogenase from dark-grown soybean seedlings[J]. Plant Physiol,1996,112(3):1357−1364. doi: 10.1104/pp.112.3.1357
[59] Fontaine JX,Tercé-Laforgue T,Bouton S,Pageau K,Lea PJ,et al. Further insights into the isoenzyme composition and activity of glutamate dehydrogenase in Arabidopsis thaliana[J]. Plant Signal Behav,2013,8(3):e23329. doi: 10.4161/psb.23329
[60] Miflin BJ,Lea PJ. Pathway of nitrogen assimilation in plants[J]. Phytochemistry,1976,15(6):873−885. doi: 10.1016/S0031-9422(00)84362-9
[61] Yang XY,Wang XF,Wei M,Hikosaka S,Goto E. Response of ammonia assimilation in cucumber seedlings to nitrate stress[J]. J Plant Biol,2010,53(3):173−179. doi: 10.1007/s12374-010-9096-9
[62] Liu Y,von Wirén N. Ammonium as a signal for philological and morphological responses in plants[J]. J Exp Bot,2017,68(10):2581−2592. doi: 10.1093/jxb/erx086
[63] Sato S,Yanagisawa S. Characterization of metabolic states of Arabidopsis thaliana under diverse carbon and nitrogen nutrient conditions via targeted metabolomic analysis[J]. Plant Cell Physiol,2014,55(2):306−319. doi: 10.1093/pcp/pct192
[64] Britto DT,Kronzucker HJ. NH4+ toxicity in higher plants:a critical review[J]. J Plant Physiol,2002,159(6):567−584. doi: 10.1078/0176-1617-0774
[65] Cao T,Xie P,Li ZQ,Ni LY,Zhang M,Xu J. Physiological stress of high NH4+ concentration in water column on the submersed macrophyte Vallisneria natans L.[J]. Bull Environ Contam Toxicol,2009,82(3):296−299. doi: 10.1007/s00128-008-9531-5
[66] Gao JQ,Li LS,Hu ZY,Yue H,Zhang RQ,Xiong ZT. Effect of ammonia stress on nitrogen metabolism of Ceratophyllum demersum[J]. Environ Toxicol Chem,2016,35(1):205−211. doi: 10.1002/etc.3182
[67] Zhou QY,Gao JQ,Zhang RM,Zhang RQ. Ammonia stress on nitrogen metabolism in tolerant aquatic plant-Myriophyllum aquaticum[J]. Ecotoxicol Environ Saf,2017,143:102−110. doi: 10.1016/j.ecoenv.2017.04.016
[68] Xian L,Zhang YZ,Cao Y,Wan T,Gong YB,et al. Glutamate dehydrogenase plays an important role in ammonium detoxification by submerged macrophytes[J]. Sci Total Environ,2020,722:137859. doi: 10.1016/j.scitotenv.2020.137859
[69] Ochieng WA,Xian L,Nasimiyu AT,Muthui SW,Ndirangu LN,et al. Exploring the ammonium detoxification mechanism of young and mature leaves of the macrophyte Potamogeton lucens[J]. Aquat Toxicol,2021,237:105879. doi: 10.1016/j.aquatox.2021.105879
[70] Wang R,Xu SJ,Jiang CC,Sun HS,Feng SG,et al. Transcriptomic sequencing and co-expression network analysis on key genes and pathways regulating nitrogen use efficiency in Myriophyllum aquaticum[J]. Int J Mol Sci,2019,20(7):1587. doi: 10.3390/ijms20071587



 下载:
下载: