Effects of drought and nitrogen application on the growth and chlorophyll fluorescence characteristics of Dalbergia odorifera T. Chen - Hevea brasiliensis Muell. Arg seedlings
-
摘要:
以降香黄檀(Dalbergia odorifera T. Chen)和橡胶树(Hevea brasiliensis Muell. Arg)为研究材料,探究干旱、施氮联合处理对幼苗生长、叶绿素荧光特性及两者互作效应的影响。结果显示,同一氮素水平下,干旱胁迫显著提高了幼苗的初始荧光(Fo)和非光化学猝灭系数(NPQ),但降低了株高增量、基径、叶长、总叶绿素含量、PSⅡ潜在活性(Fv / Fo)、PSⅡ最大光化学效率(Fv / Fm)和电子传递速率(ETR)。同一水分水平下,施氮处理组植株有更好的生长态势,且对降香黄檀的促进作用强于橡胶树;干旱与施氮联合处理显著影响降香黄檀的分支数和橡胶树的株高增量、叶柄长、叶绿素a含量、总叶绿素含量及类胡萝卜素含量。混栽对降香黄檀和湿润条件下的橡胶树有促进作用,但在干旱条件下降香黄檀显著抑制橡胶树的生长和光合性能。研究结果表明施氮可减轻干旱胁迫对两树种的不利影响,且均在施氮湿润处理组下具有最佳的生长、光合色素积累状况。此外,土壤水分变化改变了降香黄檀-橡胶树的互作效应。
Abstract:Dalbergia odorifera T. Chen and Hevea brasiliensis Muell. Arg were used to explore the effects of drought and nitrogen application on the growth, chlorophyll fluorescence characteristics, and interaction effects of the seedlings. Results showed that under the same nitrogen level, drought stress significantly increased the fluorescence parameters Fo and NPQ of the seedlings, but decreased plant height, basal diameter, leaf length, total chlorophyll, Fv/Fo, Fv/Fm, and ETR. Under the same water level, plants in the nitrogen application group exhibited better growth performance, and the promotion effects on D. odorifera were stronger than that on H. brasiliensis. Combined drought treatment and nitrogen application significantly affected the number of branches in D. odorifera and plant height increment, petiole length, chlorophyll a content, total chlorophyll content, and carotenoid content in H. brasiliensis. Mixed planting promoted the growth and development of D. odorifera and H. brasiliensis under humid conditions, while D. odorifera significantly inhibited the growth and photosynthetic performance of H. brasiliensis under drought conditions. These results showed that nitrogen application could alleviate the adverse effects of drought stress on the two species, and plant growth and photosynthetic pigment accumulation showed the best performance under nitrogen application in well-water. In addition, variations in soil moisture could change the interaction effects between D. odorifera and H. brasiliensis.
-
1. 沉水植物及其重要性
沉水植物是水体生态系统中不可或缺的一部分,在关键界面上扮演着重要角色,对湖泊的生产力和关键生源要素产生显著影响[1],对维持生态系统的结构和功能具有重要作用[2, 3]。氮(N)素对植物的生长和繁殖至关重要[4],植物可以从周围环境中吸收大量的氮素,并利用其进行氨基酸、蛋白质、DNA和其他含氮化合物的生物合成[5]。近年来,营养物质对水域生态系统健康的影响受到人们的广泛关注[6-8]。研究表明,高浓度的氮不仅会改变水域生态系统的结构,还会加速水体从清澈的稳定状态转变为浑浊的稳定状态[5, 7, 9, 10],进而对生态系统的功能产生不利影响。氮是植物生长发育所需的矿质元素,沉水植物能够通过多种方式(如直接吸收、通过构建稳定的植物-微生物自净系统等)有效降低水体的氮浓度,改善水体的营养水平,使湖泊生态系统维持稳定的健康状态[11, 12]。
2. 无机氮
氮对植物的生长发育具有重要作用,在植物生命周期中至关重要[13]。陆生植物不仅能够通过根从土壤中吸收氮,其叶片也具有氮吸收能力[14]。但由于大气中的氮主要以N2分子的形式存在,氮原子间的共价键具有较高的键能,很难被破坏[15]。自然界中只有部分微生物(如固氮微生物)和大气放电作用才能将空气中的氮气转化为植物可利用的氮[13, 15]。因此,传统观念认为陆生植物主要通过根部从土壤中吸收氮,氮进入植物细胞后,一部分被运输到地上部进行生物合成,剩余部分直接在根部被合成小分子氨基酸或储存在液泡中[16]。
硝态氮(NO3−-N)是环境中无机氮的主要形态之一,由于其很难与土壤形成表面复合物,因此在环境中容易流失[17]。土壤中的NO3−-N浓度波动较大,为了适应其浓度变化,植物形成了两种转运吸收系统:高亲和力转运系统(HATS)和低亲和力转运系统(LATS)[18]。NO3−-N在两种转运系统的作用下通过主动运输进入植物体,其中一部分会在细胞质中被还原,并同化为氨基酸,而另一部分可储备在细胞的液泡中[19]。根系吸收的氮素可通过木质部在蒸腾作用的拉力下向上运输,然后在地上部分的细胞中通过各种方式被植物同化[20]。
与NO3−-N相比,环境中的铵态氮(NH4+-N)更容易被植物吸收。植物吸收的NO3−-N可在酶促反应下还原为NH4+-N,植物也可以直接从环境中吸收NH4+-N,在此基础上进行同化等生理过程。因此,在植物体中,NH4+-N的同化比NO3−-N更加节能[20, 21]。然而,NH4+-N是一把双刃剑,其在低浓度时能促进植物的正常生理代谢,有效提高植物的生长繁殖能力。但当其浓度过高时,也会对植物产生严重的胁迫作用[22],且NH4+-N对植物的胁迫效应和毒性高于NO3−-N [8, 23, 24]。
3. 沉水植物对无机氮的吸收与运输
对于无机氮的吸收与运输,在陆生植物中的研究已经深入到分子机理层面。研究表明,对于NO3−-N的吸收与运输,目前在拟南芥(Arabidopsis thaliana (L.) Heynh.)中已发现NRT1和NRT2两大基因家族[19, 20, 23]。当环境中的NO3−处于较高浓度时,植物体内氮的吸收及运输主要受NRT1基因家族的主导,通过对LATS的调控,降低对环境中氮的吸收,以避免高氮对植物的抑制作用。研究表明,NRT1.1不仅参与植物对环境中NO3−-N吸收转运的调控,还能调控气孔的开放[21]、种子的休眠[25]、生长素的分泌[26]等代谢过程。与NRT1不同的是,NRT2的表达不受NO3−-N的诱导,当环境中NO3−的浓度较低,植物处于缺氮条件时,NRT2家族主要对HATS进行调控。在拟南芥中,NRT2.1和NRT2.2在HATS中起主要作用[27]。NRT2.4的表达受NO3−-N浓度的调控,对NO3−-N的响应浓度较广。当环境中NO3−-N不足时,植物NRT2.4在根及嫩枝中均能起到NO3−-N的转运作用[28]。
尽管环境中铵态氮的浓度通常低于硝态氮,但植物更偏向于吸收铵态氮 [21, 29]。为了进入植物细胞,植物根部的铵态氮需要依靠细胞膜上的特异性转运蛋白[30]。研究发现,当环境中NH4+-N的浓度低于0.5 mmol/L时,植物主要通过HATS进行铵的吸收和转运;当浓度超过0.5 mmol/L时,植物则改变策略,通过LATS对铵进行吸收及转运[31]。在拟南芥中发现,AMT1.1、AMT1.2、AMT1.3等基因主要负责根部NH4+-N的吸收[32, 33]。其中,AMT1.2在根部皮质层和内皮层中高表达,而AMT1.3主要在表皮层和皮质层中大量表达[32, 34]。AMT1.3的表达与外界NH4+-N的浓度密切相关[35],且受光照强度的影响,因此被认为参与植物C-N平衡的调节[35]。此外,在毛果杨(Populus trichocarpa Torr. & A. Gray ex Hook)和水稻(Oryza sativa L.)的基因组中分别发现14个和10个AMT相关基因[36, 37]。NH4+-N的吸收受多个AMT基因的协同调控,且这种调控受植物自身及其生存环境的影响[4]。沉水植物作为湖泊生态系统中重要的生物类群,不仅是湖泊生态系统多样性的基础,也是其健康运转的关键,在决定水生态系统的健康和功能上具有重要作用[34, 38, 39]。
关于沉水植物对氮的吸收,现有报道多从吸收动力学的角度展开研究。Xiong[40]发现伊乐藻(Elodea nuttallii Michx.)和苦草(Vallisneria natans (Lour.) H. Hara)对氮的吸收与水体中的磷浓度密切相关,随磷浓度的增加,两种沉水植物对NH4+-N和NO3−-N的最大吸收速率均呈下降趋势,但对NH4+-N吸收的Km值均呈上升趋势;两种植物对NO3−-N吸收的Km值趋势相反,在伊乐藻中为下降趋势,而在苦草中则呈上升趋势。Olesen等[41]研究了4种沉水植物(穿叶眼子菜(Potamogeton perfoliatus L.)、钝叶眼子菜(P. obtusifolius Mert. & W. D. J. Koch)、加拿大伊乐藻(E. canadensis Michx.)和小水毛茛(Ranunculus aquatilis L.))对不同氮素的吸收动力学。结果发现,钝叶眼子菜的Vmax最大,穿叶眼子菜和加拿大伊乐藻的较低。对苦草和黑藻(Hydrilla verticillata (L. f.) Royle)铵态氮吸收动力学的研究发现,苦草对铵态氮具有更高的吸收效率[42]。研究表明,吸收效率与外界水环境浓度密切相关,当NH4+-N的浓度为3 mg/L左右时,圆叶节节菜(Rotala rotundifolia (Buch.-Ham. ex Roxb.) Koehne)对其具有最高的吸收效率[43]。然而,也有研究指出,沉水植物对铵态氮的吸收速率随外界NH4+-N浓度的升高呈先上升后下降的趋势[44]。
目前,对沉水植物氮吸收的研究主要集中在吸收动力学,少量报道开始利用转录组分析从分子水平对其调控机理开展探讨。沉水植物通过铵转运蛋白家族(AMTs)实现水体铵态氮的吸收和转运,在NH4+-N浓度较低的环境条件下,粉绿狐尾藻(Myriophyllum aquaticum (Vell.) Verdc.)编码铵转运蛋白的基因AMT1.2和AMT3.1的表达显著上调,而当环境中NH4+-N的浓度增加时,基因的表达显著下调[45]。
沉水植物既可以通过根部从环境中吸收氮,也可以通过叶片等部位直接吸收水体中游离的氮。而陆生植物所需要的氮源主要通过根部的吸收、转运和同化[14],叶片仅能通过固氮菌实现极少量的氮固定,叶片中的氮主要来自根的向上运输过程。因此,沉水植物与陆生植物的叶片在氮的利用上可能存在显著差异,且氮在沉水植物体内的运输也可能与陆生植物不同[46]。陆生植物通常以自下而上的单向运输为主,当根部接收到高浓度氮源时,可以通过低亲和力转运系统减少根部对外源氮的吸收,或者将根部吸收的氮转移至地上部分,以降低高浓度氮对植物根系的毒害作用。当处于氮限制环境条件时,可以通过高亲和力转运系统增加根部氮的吸收,并向上运输,以满足地上部分的氮需求[47, 48]。沉水植物由于地下和地上部分均能获取环境中的氮[49-51],为了适应水环境中氮含量的变化,其对氮的运输存在向上及向下的双向运输。为了减轻高浓度氮对自身的毒害,沉水植物对氮的利用在地上部分(叶等器官)和地下部分(根或根茎)存在一定的权衡关系。我们近期的研究表明,沉水植物对外源铵的转运存在明显的双向运输(图1)。当环境中的氮含量受限时,沉水植物主要通过地下部分从沉积物中吸收氮,并向上运输至地上部分;而当环境中的铵浓度过高时,沉水植物的双向运输依然存在,其地上部分能够通过迅速的氮合成显著降低植物体中游离氮的积累,从而避免毒害作用的产生[42]。
![]() 图 1 沉水植物在不同铵氮浓度条件下的吸收及运输过程(改自Xian等[46])箭头宽度代表植物地上及地下部分对外源铵态氮吸收及转运相对量的大小。GS:谷氨酰胺合成酶,GDH:谷氨酸脱氢酶,Glu:谷氨酸,Gln:谷氨酰胺。红色代谢途径为当前环境条件下植物铵同化的主要途径。Figure 1. Ammonium uptake and transportation in submerged plants under different ammonium concentrations (modified from Xian et al.[46])Arrow width represents relative amount of external ammonium uptake and transport by aboveground and underground parts of submerged macrophytes. GS: glutamine synthetase, GDH: glutamate dehydrogenase, Glu: glutamate, Gln: glutamine. Red metabolic pathways indicate primary route for ammonium assimilation under current environmental conditions.
图 1 沉水植物在不同铵氮浓度条件下的吸收及运输过程(改自Xian等[46])箭头宽度代表植物地上及地下部分对外源铵态氮吸收及转运相对量的大小。GS:谷氨酰胺合成酶,GDH:谷氨酸脱氢酶,Glu:谷氨酸,Gln:谷氨酰胺。红色代谢途径为当前环境条件下植物铵同化的主要途径。Figure 1. Ammonium uptake and transportation in submerged plants under different ammonium concentrations (modified from Xian et al.[46])Arrow width represents relative amount of external ammonium uptake and transport by aboveground and underground parts of submerged macrophytes. GS: glutamine synthetase, GDH: glutamate dehydrogenase, Glu: glutamate, Gln: glutamine. Red metabolic pathways indicate primary route for ammonium assimilation under current environmental conditions.4. 沉水植物对无机氮的同化
植物氮同化是合成含氮有机化合物的关键步骤,一般而言,该步骤涉及多种酶的参与,包括硝酸还原酶(NR)、亚硝酸还原酶(NiR)、谷氨酰胺合成酶(GS)、谷氨酸合成酶(GOGAT)和谷氨酸脱氢酶(GDH)等。植物吸收硝态氮后,一部分储存在木质部,或者芽和根等器官的液泡中;另一部分在细胞质中被NR还原为NO2–,后者被植物细胞迅速运输至质体中还原,最终以NH4+的形式被植物同化[33, 52]。植物体内的NH4+-N主要通过GS-GOGAT(GS/GOGAT循环途径)被同化(图2)。其中,GS对NH4+有较高的亲和力,作为氮代谢过程的关键酶,能够将铵转化为谷氨酰胺,后者在GOGAT的作用下结合一分子的谷氨酸,与α-酮戊二酸(2-OG)结合,产生两分子的谷氨酸[53-55]。植物体中的GOGAT有两种亚型:Fd-GOGAT和NADH-GOGAT,前者主要从还原性铁中获取电子[56],而后者则主要依赖呼吸作用产生的NADH提供电子[57]。除了GS-GOGAT循环, GDH催化的代谢途径也被认为是植物铵同化的一条途径。GDH在植物体内广泛存在,是一种分子量为208~300 kDa的蛋白质[58],通常形成六聚体[59]。它能催化NH4+和α-酮戊二酸结合生成谷氨酸,同时也能催化谷氨酸的分解(图2)。在高等植物中,GDH分为两种类型:以NADPH为电子供体的NADPH-GDH存在于叶绿体中,而以NADH为电子供体的NADH-GDH则存在于线粒体中[59]。尽管早期研究普遍认为GDH催化的代谢途径是植物进行铵同化的主要途径,但到了1976年,由于GOGAT的发现,GS-GOGAT循环才被认为是高等植物同化铵的主要途径[60]。目前,通过对GS-GOGAT循环和GDH途径的深入探讨,研究者更深入地比较了植物中铵同化途经的特征。如,在铵胁迫条件下,黄瓜(Cucumis sativus L.)叶片主要通过GS-GOGAT循环途经同化铵,而根部则主要通过GDH途经来同化[61]
![]() 图 2 植物无机氮同化路径GS:谷氨酰胺合成酶;GDH:谷氨酸脱氢酶;GOGAT:谷氨酸合成酶;Glu:谷氨酸;Gln:谷氨酰胺;2-OG:α-酮戊二酸。红色和黑色分别代表两条无机氮同化途径:黑色为GS/GOGAT循环途径,红色为GDH途径。Figure 2. Inorganic nitrogen assimilation pathways in plantsGS: Glutamine synthetase; GDH: Glutamate dehydrogenase; GOGAT: Glutamate synthase; Glu: Glutamate; Gln: Glutamine; 2-OG: α-ketoglutarate. Red and black represent two pathways to inorganic nitrogen assimilation: black denotes GS/GOGAT cycle pathway, red denotes GDH pathway.
图 2 植物无机氮同化路径GS:谷氨酰胺合成酶;GDH:谷氨酸脱氢酶;GOGAT:谷氨酸合成酶;Glu:谷氨酸;Gln:谷氨酰胺;2-OG:α-酮戊二酸。红色和黑色分别代表两条无机氮同化途径:黑色为GS/GOGAT循环途径,红色为GDH途径。Figure 2. Inorganic nitrogen assimilation pathways in plantsGS: Glutamine synthetase; GDH: Glutamate dehydrogenase; GOGAT: Glutamate synthase; Glu: Glutamate; Gln: Glutamine; 2-OG: α-ketoglutarate. Red and black represent two pathways to inorganic nitrogen assimilation: black denotes GS/GOGAT cycle pathway, red denotes GDH pathway.对于沉水植物而言,由于水体中的无机氮主要以NO3−-N和NH4+-N的形式存在,植物更倾向于利用NH4+-N,因为消耗的能量更少[62, 63]。沉水植物的氮同化过程与陆生植物类似,但在同化部位、同化路径的选择等方面有较大差异。由于直接受水体氮浓度波动的影响,沉水植物主要通过叶片进行氮素的同化和利用[42]。从环境中吸收氮后,沉水植物通常会将其合成含氮有机物储存在体内,以避免氮在体内积累导致的毒害[64, 65]。目前,相对于陆生植物,对沉水植物氮同化的机制研究还不够深入,其氮同化途径的特征仍不明确。仅有少量报道比较了胁迫条件下水生植物铵同化过程中关键酶的活性。譬如,水体中NH4+-N的浓度和pH值显著影响金鱼藻(Ceratophyllum demersum L.)GS的活性,在pH值为9的溶液中,GS活性显著下降;同样,在高浓度NH4+-N条件下,GS活性随着胁迫时间的增加也呈显著下降趋势[66]。粉绿狐尾藻也表现出类似的趋势,当环境中NH4+-N浓度过高时,其叶片中GS的活性被显著抑制,但当NH4+-N浓度为120 mg/L时,叶片中NR的活性迅速上升[67]。转录组分析发现,随着水体NH4+-N浓度的升高,粉绿狐尾藻叶片中编码GS的关键基因的表达量显著下降,而多个GDH和GOGAT的编码基因的表达则显著上调[61]。
最近研究发现,沉水植物对铵的同化特征受物种及生长时期的影响。在高浓度铵态氮条件下,铵耐受型物种穗花狐尾藻(M. spicatum L.)主要通过GDH途径进行铵的同化作用[46, 68],而铵敏感型物种光叶眼子菜(P. lucen L.)主要通过GS-GOGAT途径对铵进行同化(图3:A、B)[69]。针对同一物种不同生长时期的研究发现,沉水植物幼叶主要通过GDH途径同化水体中的铵态氮,而成熟叶在正常浓度条件下主要通过GS-GOGAT途径实现铵态氮的同化(图3:C、D)[69]。此外,比较转录组分析结果进一步表明,沉水植物光叶眼子菜谷氨酸脱氢酶基因GDH2在不同浓度铵态氮条件下的表达量存在显著差异。
![]() A:高浓度铵态氮条件下,耐受型物种穗花狐尾藻铵同化以GDH途径为主导;B:敏感型物种光叶眼子菜铵同化以GS/GOGAT途径为主导; C:光叶眼子菜幼叶主要通过GDH途径进行铵的同化;D:成熟叶主要通过GS/GOGAT循环进行铵的同化。缩写同图2。Figure 3. Inorganic nitrogen assimilation pathways in submerged macrophytes with different adaptive capacities and developmental stages (modified from Xian et al.[46, 68] and Ochieng et al.[69])A: Ammonium assimilation in ammonium-tolerant species M. spicatum is dominated by GDH pathway under high ammonium concentration. B: Ammonium assimilation in ammonium-sensitive species P. lucens is dominated by GS/GOGAT pathway. C: In young leaves of P. lucens, ammonium assimilation is primarily mediated through the GDH pathway. D: In mature leaves of P. lucens, ammonium assimilation is mainly mediated through the GS/GOGAT cycle. Abbreviations are as in Fig. 2.
A:高浓度铵态氮条件下,耐受型物种穗花狐尾藻铵同化以GDH途径为主导;B:敏感型物种光叶眼子菜铵同化以GS/GOGAT途径为主导; C:光叶眼子菜幼叶主要通过GDH途径进行铵的同化;D:成熟叶主要通过GS/GOGAT循环进行铵的同化。缩写同图2。Figure 3. Inorganic nitrogen assimilation pathways in submerged macrophytes with different adaptive capacities and developmental stages (modified from Xian et al.[46, 68] and Ochieng et al.[69])A: Ammonium assimilation in ammonium-tolerant species M. spicatum is dominated by GDH pathway under high ammonium concentration. B: Ammonium assimilation in ammonium-sensitive species P. lucens is dominated by GS/GOGAT pathway. C: In young leaves of P. lucens, ammonium assimilation is primarily mediated through the GDH pathway. D: In mature leaves of P. lucens, ammonium assimilation is mainly mediated through the GS/GOGAT cycle. Abbreviations are as in Fig. 2.5. 展望
近年来,尽管沉水植物氮的吸收与利用受到了广泛关注,但仍需在不同物种(广度)及不同研究层次(深度)上开展细致的研究。
沉水植物的叶片能够利用水体的无机氮,并在细胞内通过一系列酶促反应最终形成谷氨酰胺和谷氨酸。我们近期通过代谢通路关联性分析发现,产生的谷氨酸能够在植物体内参与多种代谢过程,进而被植物高效利用,在众多代谢通路中,关系最紧密的是植物的光合碳同化(图4):(1)沉水植物铵同化过程中产生的谷氨酰胺能与α-酮戊二酸结合,在谷氨酸合成酶的催化作用下生成谷氨酸;(2)谷氨酸脱氢酶能够直接催化2-OG与铵结合,并生成谷氨酸;(3)谷氨酸和草酰乙酸(OAA)能够在谷草转氨酶(GOT)的作用下生成天冬氨酸和α-酮戊二酸。在此过程中,沉水植物主要通过α-酮戊二酸和草酰乙酸将碳氮代谢进行关联。然而,在不同氮素浓度条件下,沉水植物通过何种途径实现2-OG 和OAA在碳氮联合利用过程中的调控还不清楚,相关机制还需要深入研究和发掘。
尽管对沉水植物氮的吸收、运输和同化过程等方面开展了相关探讨,但研究深度还远远不够。目前仅在生理响应层面,通过酶活性的变化,揭示了无机氮同化中的关键代谢途径[68, 69],但对这些关键酶的活性及其基因表达的调控方式还缺乏相应研究,目前仅有少量报道从转录组层面进行了分析[70]。研究难以深入的原因主要是沉水植物尚未构建成熟的遗传转化系统,且缺乏模式植物。此外,尽管沉水植物的基因组总体较小,但仅有少量物种的全基因组序列被发表[20]。因此,还需进一步探索研究方法,构建合适的遗传转化体系,同时采取最新的基因编辑等分子技术,从而实现对沉水植物功能基因的验证,以及对关键蛋白质结构和功能的深入研究。
-
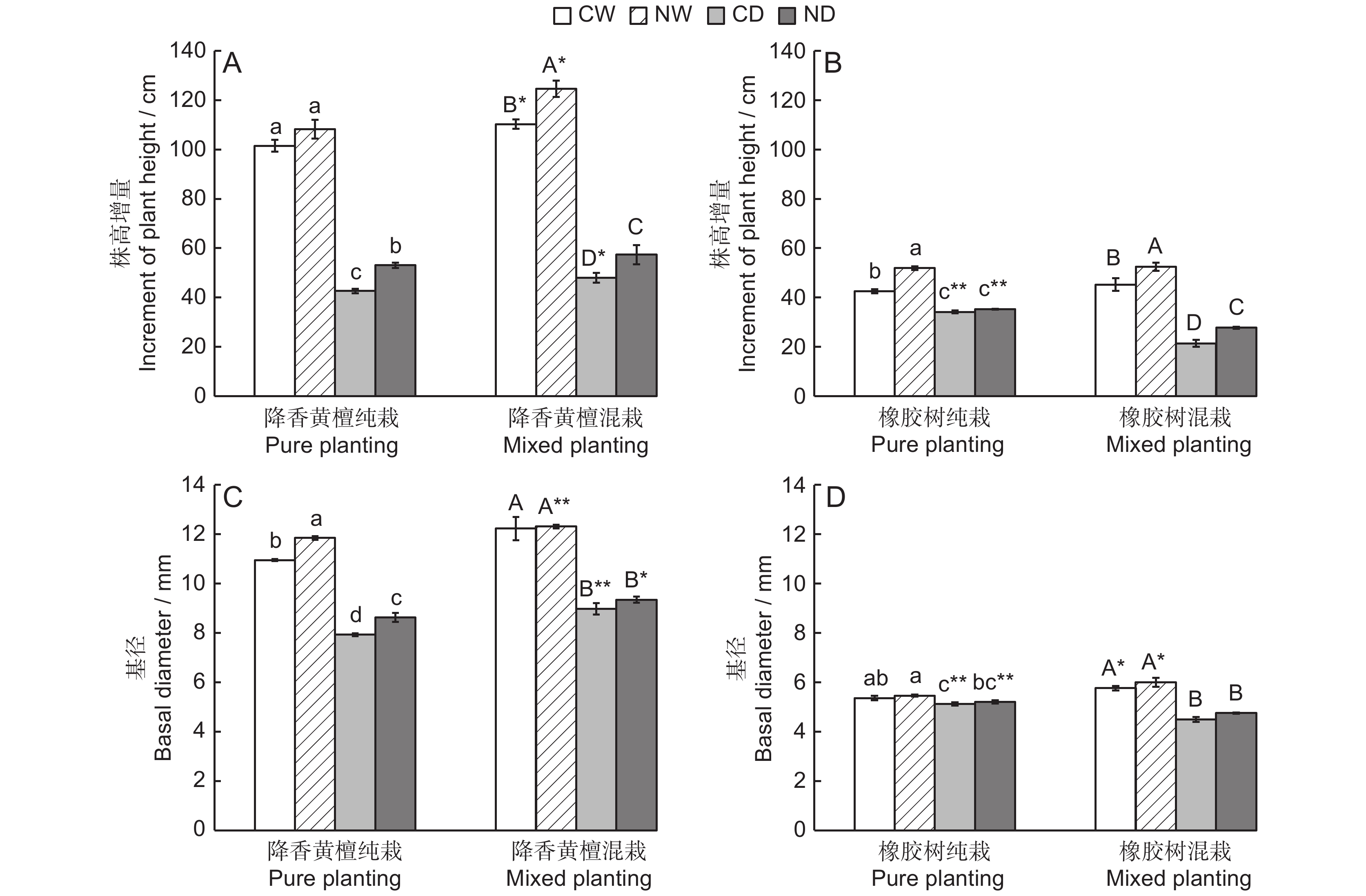
图 1 不同处理下降香黄檀和橡胶树幼苗的株高增量、基径差异
不同大小写字母分别代表混栽和纯栽幼苗不同处理间差异显著(P < 0.05)。* 和** 分别表示同一处理下,不同栽植模式的降香黄檀或橡胶树幼苗在P < 0.05和P < 0.01水平上差异显著、极显著。下同。
Figure 1. Differences in plant height increment and basal diameter of Dalbergia odorifera and Hevea brasiliensis seedlings under different treatments
Different uppercase/lowercase letters represent significant differences between different treatments of mixed and pure seedlings, respectively (P < 0.05). * and ** indicate significant difference and extremely significant difference at P < 0.05 and P < 0.01 levels in D. odorifera or H. brasiliensis seedlings with different planting patterns under the same treatment, respectively. Same below.
表 1 栽植模式、水分和氮素及其交互作用对两树种株高增量、基径的影响
Table 1 Effects of planting pattern, water, nitrogen, and their interactions on height increment and basal diameter of two tree species
物种
Species参数
ParameterC W N C × W C × N W × N C × W × N 降香黄檀
Dalbergia odorifera株高增量 0.000** 0.000** 0.000** 0.052 0.391 0.870 0.257 基径 0.000** 0.000** 0.002** 0.976 0.061 0.874 0.413 橡胶树
Hevea brasiliensis株高增量 0.000** 0.000** 0.000** 0.000** 0.401 0.021* 0.057 基径 0.596 0.000** 0.018* 0.000** 0.236 0.978 0.948 注:C,栽植模式;W,水分水平;N,氮素水平。表中数值为P值。*,P < 0.05;**,P < 0.01。
Notes: C, planting pattern; W, water level; N, nitrogen level. Values are P-values.表 2 不同处理条件对降香黄檀和橡胶树幼苗叶片性状的影响
Table 2 Effects of different treatments on leaf traits of Dalbergia odorifera and Hevea brasiliensis seedlings
栽植模式
Planting pattern处理
Treatment降香黄檀
D. odorifera橡胶树
H. brasiliensis叶长
LL / cm叶宽
LW / cm分支数增量
INB / twig/plant总叶面积
TA / cm2叶长
LL / cm叶宽
LW / cm叶柄长
LPL / cm平均单叶面积
ALA / cm2纯栽 CW 6.18 ± 0.08b 2.86 ± 0.09b 24.88 ± 0.52b 4605.05 ± 179.48b 8.58 ± 0.26a 2.90 ± 0.07a 7.68 ± 0.27b 7.92 ± 0.64a NW 6.73 ± 0.10a 3.20 ± 0.07a 34.00 ± 1.19a 6044.16 ± 630.23a 10.31 ± 1.04a 3.03 ± 0.23a 9.13 ± 0.13a 9.37 ± 0.94a CD 5.50 ± 0.12c 2.55 ± 0.06c 13.38 ± 0.47d 1680.67 ± 48.67c 8.43 ± 0.60a 2.93 ± 0.14a 7.50 ± 0.35b 7.70 ± 1.18a* ND 6.50 ± 0.26ab 2.88 ± 0.09b 18.13 ± 0.43c 2558.30 ± 128.05c 9.08 ± 0.20a 2.94 ± 0.13a 7.43 ± 0.15b 7.80 ± 0.91a 混栽 CW 6.44 ± 0.15B 3.16 ± 0.11B 35.00 ± 0.91B** 4842.23 ± 301.98B 8.98 ± 0.21B 2.98 ± 0.12A 7.63 ± 0.47B 8.18 ± 1.00AB NW 7.66 ± 0.33A* 3.40 ± 0.15A 41.75 ± 1.25A** 6178.16 ± 265.31A 11.06 ± 0.22A 3.10 ± 0.15A 9.78 ± 0.65A 10.38 ± 3.17A CD 5.29 ± 0.11C 2.53 ± 0.08C 17.00 ± 0.41C** 2360.02 ± 44.76C** 8.00 ± 0.29C 2.79 ± 0.06A 6.74 ± 0.51B 3.39 ± 0.45B ND 6.30 ± 0.11B 2.89 ± 0.08B 18.25 ± 0.25C 2659.07 ± 139.59C 8.93 ± 0.22B 2.91 ± 0.09A 7.38 ± 0.24B 6.00 ± 0.67AB FC 0.129 0.076 0.000** 0.160 0.575 0.947 0.848 0.227 FW 0.000** 0.000** 0.000** 0.000** 0.003** 0.268 0.000** 0.010* FN 0.000** 0.000** 0.000** 0.000** 0.001** 0.314 0.001** 0.117 FC × W 0.004** 0.058 0.000** 0.611 0.253 0.394 0.210 0.070 FC × N 0.182 0.770 0.008** 0.399 0.735 0.761 0.210 0.410 FW × N 0.639 0.659 0.000** 0.056 0.081 0.761 0.011* 0.811 FC × W × N 0.198 0.618 0.660 0.555 0.849 0.761 0.991 0.659 注:表中数据为平均值 ± 标准误。上方数据不同大/小写字母分别代表混栽和纯栽幼苗不同处理间差异显著(P < 0.05)。* 和** 分别表示同一处理下不同栽植模式的降香黄檀或橡胶树幼苗在P < 0.05和 P < 0.01水平上差异显著。下方数据为栽植模式(C)、水分(W)、氮素(N)及其交互作用对降香黄檀和橡胶树幼苗各指标的影响,表中数值为P值。*,P < 0.05;**,P < 0.01。 Notes: Data in the table are Mean ± Standard error. Upper data, different uppercase/lowercase letters represent significant differences between different treatments of mixed and pure seedlings, respectively (P < 0.05). *, significant differences in D. odorifera or H. brasiliensis seedlings with different planting patterns under the same treatment (P < 0.05); **, extremely significant differences in D. odorifera and H. brasiliensis seedlings with different planting patterns under the same treatment (P < 0.01). Below data, effects of planting pattern (C), water (W), nitrogen (N), and their interactions on various indicators of D. odorifera and H. brasiliensis seedlings. Values are P-values. *, P < 0.05; **, P < 0.01. 表 3 不同处理条件对降香黄檀和橡胶树幼苗光合色素含量的影响
Table 3 Effects of different treatment conditions on content of photosynthetic pigments in Dalbergia odorifera and Hevea brasiliensis seedlings
栽植模式
Planting
pattern处理
Treatment降香黄檀
D. odorifera橡胶树
H. brasiliensis叶绿素a
Chl a / μg/g叶绿素b
Chl b / μg/g总叶绿素
Chltotal / μg/g类胡萝卜素
Caro / μg/g叶绿素a
Chl a / μg/g叶绿素b
Chl b / μg/g总叶绿素
Chltotal / μg/g类胡萝卜素
Caro / μg/g纯栽 CW 1127.30 ± 39.42ab 477.44 ± 35.77ab 1604.74 ± 75.07ab 282.10 ± 4.62a 772.41 ± 4.31b 265.86 ± 1.64a 1038.27 ± 5.03b 323.62 ± 2.40a NW 1180.34 ± 11.89a 507.18 ± 17.59a 1687.52 ± 27.86a 323.59 ± 22.72a 854.60 ± 32.33a 279.14 ± 8.36a 1117.00 ± 41.80a 336.63 ± 16.17a CD 1077.24 ± 16.13c 398.84 ± 17.35c 1476.08 ± 14.53b 285.02 ± 28.52a 656.72 ± 9.91c** 233.95 ± 3.41b** 890.67 ± 11.35c** 196.23 ± 1.15c** ND 1121.75 ± 12.11ab 433.58 ± 5.33bc 1555.33 ± 17.14ab 313.58 ± 10.48a 702.96 ± 12.28c** 240.26 ± 3.74b** 943.22 ± 14.19c** 291.15 ± 4.73b** 混栽 CW 1172.70 ± 22.3AB* 507.75 ± 15.83A 1680.45 ± 37.95AB 309.94 ± 6.62C* 887.56 ± 5.03B** 389.88 ± 9.80B** 1277.44 ± 13.64B** 331.49 ± 43.94A NW 1227.21 ± 8.80A 508.30 ± 9.91A 1735.51 ± 18.71A 385.25 ± 4.49A* 980.66 ± 6.76A** 508.08 ± 12.07A** 1468.39 ± 11.01A** 353.16 ± 4.88A CD 1151.00 ± 34.60B 429.86 ± 41.54B 1580.86 ± 76.12B 338.81 ± 5.17B 285.17 ± 11.42D 92.55 ± 5.58D 377.71 ± 16.53D 144.28 ± 4.86C ND 1166.60 ± 10.02AB* 441.54 ± 9.04AB 1608.15 ± 18.94AB 373.73 ± 15.95A* 563.26 ± 5.59C 191.34 ± 4.34C 754.60 ± 9.33C 233.36 ± 3.14B FC 0.003** 0.281 0.030* 0.000** 0.000** 0.000** 0.046* 0.086 FW 0.006** 0.000** 0.001** 0.811 0.000** 0.000** 0.000** 0.000** FN 0.014* 0.241 0.056 0.000** 0.000** 0.000** 0.000** 0.000** FC × W 0.680 0.907 0.783 0.571 0.000** 0.000** 0.000** 0.010* FC × N 0.667 0.421 0.520 0.354 0.000** 0.000** 0.000** 0.953 FW × N 0.459 0.803 0.800 0.222 0.000** 0.194 0.006** 0.005** FC × W × N 0.634 0.925 0.844 0.524 0.000** 0.535 0.000** 0.764 注:表中数据为平均值 ± 标准误。上方数据不同大/小写字母分别代表混栽和纯栽幼苗不同处理间差异显著(P < 0.05)。* 和** 分别表示同一处理下不同栽植模式的降香黄檀或橡胶树幼苗在P < 0.05和 P < 0.01水平上差异显著。下方数据为栽植模式(C)、水分(W)、氮素(N)及其交互作用对降香黄檀和橡胶树幼苗各指标的影响,表中数值为P值。*,P < 0.05;**,P < 0.01。下同。 Notes: Data in the table are Mean ± Standard error. Upper data, different uppercase/lowercase letters represent significant differences between different treatments of mixed and pure seedlings, respectively (P < 0.05). *, significant differences in D. odorifera or H. brasiliensis seedlings with different planting patterns under the same treatment (P < 0.05); **, extremely significant differences in D. odorifera and H. brasiliensis seedlings with different planting patterns under the same treatment (P < 0.01). Below data, effects of planting pattern (C), water (W), nitrogen (N), and their interactions on various indicators of D. odorifera and H. brasiliensis seedlings. Values are P-values. *, P < 0.05; **, P < 0.01. Same below. 表 4 不同处理条件对降香黄檀和橡胶树幼苗叶绿素荧光参数的影响
Table 4 Effects of different treatments on chlorophyll fluorescence parameters of Dalbergia odorifera and Hevea brasiliensis seedlings
栽植
模式
Planting pattern处理
Treatment降香黄檀
D. odorifera橡胶树
H. brasiliensis初始荧光Fo PS Ⅱ潜在活性
Fv / FoPS Ⅱ最大光化学效率Fv / Fm 电子传递速率ETR 初始荧光Fo PS Ⅱ潜在活性
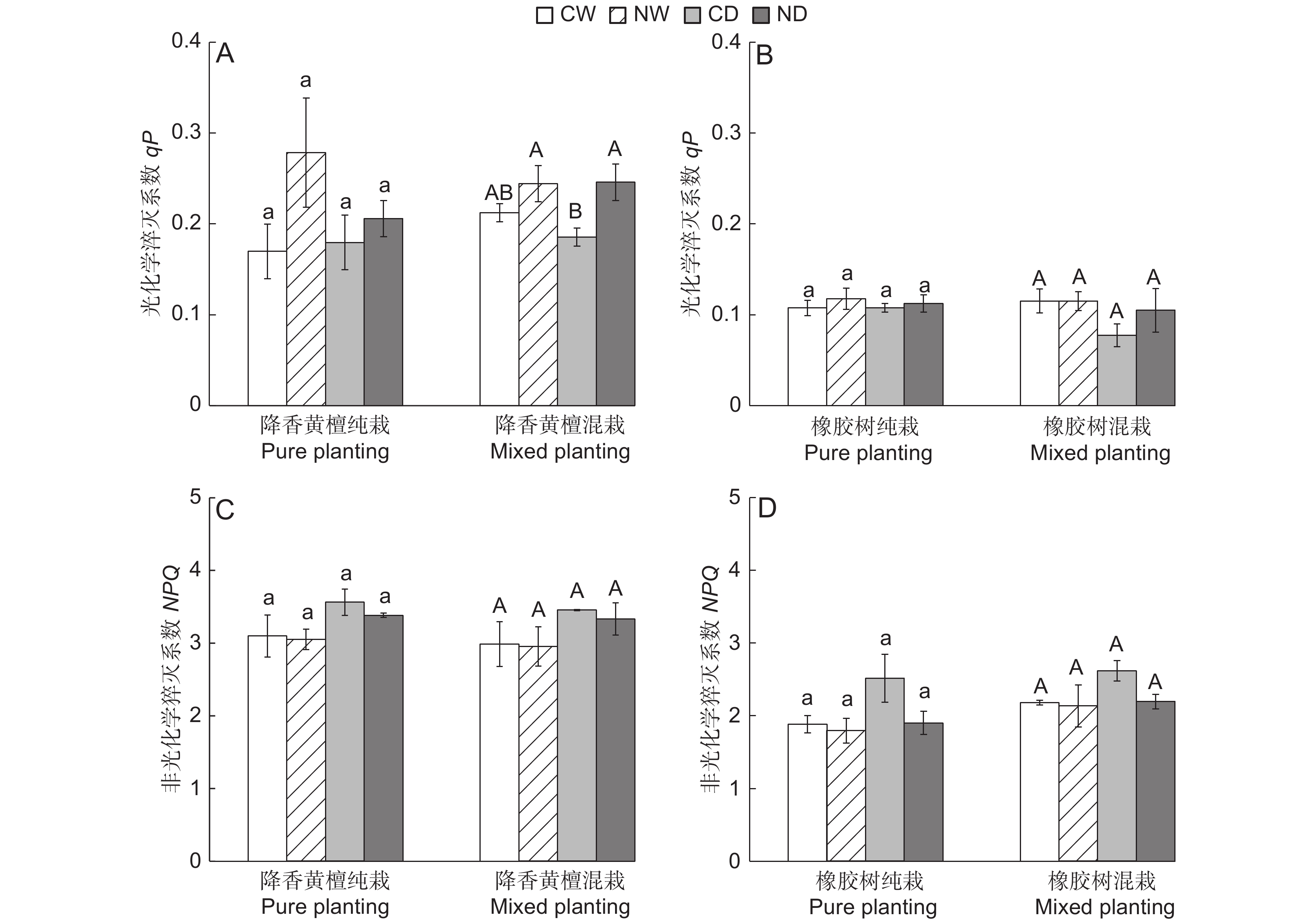
Fv / FoPS Ⅱ最大光化学效率Fv / Fm 电子传递速率ETR 纯栽 CW 354.00 ± 27.05ab 4.76 ± 0.29ab 0.82 ± 0.01a 40.48 ± 7.43b 317.75 ± 26.20a 4.14 ± 0.34ab 0.81 ± 0.01ab 33.65 ± 2.22a NW 318.75 ± 8.11b* 5.19 ± 0.13a 0.83 ± 0.00a 72.03 ± 13.44a 300.50 ± 22.48a 4.98 ± 0.21a 0.83 ± 0.01a 33.55 ± 2.20a CD 404.00 ± 38.36a 3.62 ± 0.15c 0.78 ± 0.01b 40.30 ± 4.90b 341.25 ± 34.78a 3.52 ± 0.52b 0.77 ± 0.03b 26.23 ± 1.84b ND 286.50 ± 17.05b 4.44 ± 0.13b 0.82 ± 0.00a 51.43 ± 5.86ab 337.75 ± 5.02a 4.15 ± 0.16ab 0.81 ± 0.01ab 31.68 ± 2.45ab 混栽 CW 285.50 ± 26.01A 4.84 ± 0.41AB 0.83 ± 0.01AB 53.95 ± 2.54AB 292.25 ± 14.34AB 4.43 ± 0.08AB 0.82 ± 0.00AB 31.08 ± 3.07A NW 258.75 ± 15.50A 5.47 ± 0.55A 0.84 ± 0.01A 63.85 ± 5.61A 272.50 ± 16.83B 5.19 ± 0.31A 0.84 ± 0.01A 33.05 ± 3.27A CD 321.75 ± 22.05A 3.87 ± 0.26B 0.79 ± 0.01B 45.65 ± 2.31B 381.25 ± 26.31A 3.17 ± 0.29C 0.76 ± 0.01C 19.60 ± 2.92A ND 261.00 ± 9.60A 4.76 ± 0.26AB 0.82 ± 0.00AB 60.70 ± 4.77A 328.75 ± 44.30AB 4.00 ± 0.31B 0.79 ± 0.01B 27.95 ± 6.25A FC 0.001** 0.293 0.407 0.304 0.766 0.994 0.784 0.163 FW 0.386 0.000** 0.000** 0.102 0.011* 0.000** 0.000** 0.010* FN 0.001** 0.004** 0.004** 0.002** 0.225 0.002** 0.008** 0.105 FC × W 0.747 0.807 0.710 0.628 0.269 0.251 0.450 0.443 FC × N 0.316 0.756 0.976 0.360 0.497 0.896 0.995 0.598 FW × N 0.080 0.467 0.075 0.429 0.801 0.873 0.436 0.213 FC × W × N 0.456 0.881 0.771 0.190 0.539 0.742 0.995 0.930 表 5 栽植模式、水分和氮素及其交互作用对两树种qP、NPQ的影响
Table 5 Effects of planting pattern, water, nitrogen, and their interactions on qP and NPQ of two tree species
物种
Species参数
ParameterC W N C × W C × N W × N C × W × N 降香黄檀
D. odoriferaqP 0.515 0.289 0.010* 0.650 0.608 0.515 0.187 NPQ 0.539 0.013* 0.528 0.938 0.904 0.710 0.942 橡胶树
H. brasiliensisqP 0.423 0.144 0.203 0.302 0.812 0.712 0.373 NPQ 0.073 0.035* 0.045* 0.657 0.670 0.113 0.787 -
[1] Geng SC,Chen ZJ,Han SJ,Wang F,Zhang JH. Rainfall reduction amplifies the stimulatory effect of nitrogen addition on N2O emissions from a temperate forest soil[J]. Sci Rep,2017,7:43329. doi: 10.1038/srep43329
[2] 陈亚宁,李玉朋,李稚,刘永昌,黄文静,等. 全球气候变化对干旱区影响分析[J]. 地球科学进展,2022,37(2):111−119. doi: 10.11867/j.issn.1001-8166.2022.006 Chen YN,Li YP,Li Z,Liu YC,Huang WJ,et al. Analysis of the impact of global climate change on dryland areas[J]. Advances in Earth Science,2022,37 (2):111−119. doi: 10.11867/j.issn.1001-8166.2022.006
[3] 罗海婧,张永清,石艳华,李鑫,张耀文. 不同红小豆品种幼苗对干旱胁迫的生理响应[J]. 植物科学学报,2014,32(5):493−501. doi: 10.11913/PSJ.2095-0837.2014.50493 Luo HJ,Zhang YQ,Shi YH,Li X,Zhang YW. Effects of drought stress on the physiological characteristics of different adzuki bean varieties at the seedling stage[J]. Plant Science Journal,2014,32 (5):493−501. doi: 10.11913/PSJ.2095-0837.2014.50493
[4] 孙娅楠,赵杨,赵渊祥,曹海,龙建磊. 棕榈幼苗光合和叶绿素荧光对干旱胁迫及复水的响应[J]. 中南林业科技大学学报,2021,41(9):45−52. doi: 10.14067/j.cnki.1673-923x.2021.09.005 Sun YN,Zhao Y,Zhao YX,Cao H,Long JL. Effects of drought and rewatering on photosynthetic characteristics and chlorophyll fluorescence of Trachycarpus fortunei seedlings[J]. Journal of Central South University of Forestry & Technology,2021,41 (9):45−52. doi: 10.14067/j.cnki.1673-923x.2021.09.005
[5] Babaei K,Moghaddam M,Farhadi N,Ghasemi Pirbalouti A. Morphological,physiological and phytochemical responses of Mexican marigold (Tagetes minuta L. ) to drought stress[J]. Sci Hortic,2021,284:110116. doi: 10.1016/j.scienta.2021.110116
[6] 张金凤,陈佩珍,孙晓波,胡兴峰,季孔庶. 干旱对马尾松幼苗光合作用及相关生理的影响[J]. 中国农学通报,2021,37(1):32−38. doi: 10.11924/j.issn.1000-6850.casb20200100002 Zhang JF,Chen PZ,Sun XB,Hu XF,Ji KS. Effects on photosynthetic and resistant physiological characteristics of Pinus massoniana seedlings under drought stress[J]. Chinese Agricultural Science Bulletin,2021,37 (1):32−38. doi: 10.11924/j.issn.1000-6850.casb20200100002
[7] Ren HJ,Chen YC,Wang XT,Wong GTF,Cohen AL,et al. 21st-century rise in anthropogenic nitrogen deposition on a remote coral reef[J]. Science,2017,356 (6339):749−752. doi: 10.1126/science.aal3869
[8] Schlesinger WH. On the fate of anthropogenic nitrogen[J]. Proc Natl Acad Sci USA,2009,106 (1):203−208. doi: 10.1073/pnas.0810193105
[9] 裴昊斐,高卫东,方娇阳,叶可可,祝燕,等. 模拟氮沉降对一年生香椿幼苗生长和光合特性的影响[J]. 中国生态农业学报,2019,27(10):1546−1552. Pei HF,Gao WD,Fang JY,Ye KK,Zhu Y,et al. Effects of simulated nitrogen deposition on growth and photosynthetic characteristics of one-year-old Toona sinensis seedlings[J]. Chinese Journal of Eco-Agriculture,2019,27 (10):1546−1552.
[10] 韦献东,施福军,梁小春,陆海燕,刘天泉,王凌晖. 模拟氮沉降对桢楠幼苗生长的影响[J]. 北方园艺,2020(8):74−79. Wei XD,Shi FJ,Liang XC,Lu HY,Liu TQ,Wang LH. Effects of simulated nitrogen deposition on the growth of Phoebe zhennan seedlings[J]. Northern Horticulture,2020 (8):74−79.
[11] Xiong X,Chang LY,Khalid M,Zhang JJ,Huang DF. Alleviation of drought stress by nitrogen application in Brassica campestris ssp. Chinensis L.[J]. Agronomy,2018,8 (5):66. doi: 10.3390/agronomy8050066
[12] Zhang SK,Shao L,Sun ZY,Huang Y,Liu N. An atmospheric pollutant (inorganic nitrogen) alters the response of evergreen broad-leaved tree species to extreme drought[J]. Ecotoxicol Environ Saf,2020,187:109750. doi: 10.1016/j.ecoenv.2019.109750
[13] Meng B,Shi BK,Zhong SZ,Chai H,Li SX. Drought sensitivity of aboveground productivity in Leymus chinensis meadow steppe depends on drought timing[J]. Oecologia,2019,191 (3):685−696. doi: 10.1007/s00442-019-04506-w
[14] 徐楠楠. 水分、光照和氮沉降对五种暖温带典型乔木幼苗生理生态学特性的影响[D]. 济南: 山东大学, 2015: 1−133. [15] Cheng HY,Wei M,Wang S,Wu BD,Wang CY. Atmospheric N deposition alleviates the unfavorable effects of drought on wheat growth[J]. Braz J Bot,2020,43 (2):229−238. doi: 10.1007/s40415-020-00598-4
[16] Wang S,Wei M,Wu BD,Cheng HY,Jiang K,Wang CY. Does N deposition mitigate the adverse impacts of drought stress on plant seed germination and seedling growth?[J]. Acta Oecol,2020,109:103650. doi: 10.1016/j.actao.2020.103650
[17] 蒲玉瑾,张丽佳,苗灵凤,杨帆. 不同钙离子浓度对低温下降香黄檀幼苗生长及生理特性的影响[J]. 植物科学学报,2019,37(2):251−259. doi: 10.11913/PSJ.2095-0837.2019.20251 Pu YJ,Zhang LJ,Miao LF,Yang F. Effects of different calcium concentrations on the growth and physiological characteristics of Dalbergia odorifera under low temperatures[J]. Plant Science Journal,2019,37 (2):251−259. doi: 10.11913/PSJ.2095-0837.2019.20251
[18] 郭璐瑶,苗灵凤,李大东,向丽珊,杨帆. 施氮和增温对降香黄檀幼苗生长发育和生理特征的影响[J]. 植物科学学报,2022,40(2):259−268. doi: 10.11913/PSJ.2095-0837.2022.20259 Guo LY,Miao LF,Li DD,Xiang LS,Yang F. Effects of nitrogen addition and warming on growth,development,and physiological characteristics of Dalbergia odorifera T. Chen seedlings[J]. Plant Science Journal,2022,40 (2):259−268. doi: 10.11913/PSJ.2095-0837.2022.20259
[19] 李国尧,王权宝,李玉英,周双喜,于海英. 橡胶树产胶量影响因素[J]. 生态学杂志,2014,33(2):510−517. doi: 10.13292/j.1000-4890.2014.0036 Li GY,Wang QB,Li YY,Zhou SX,Yu HY. A review of influencing factors on latex yield of Hevea brasiliensis[J]. Chinese Journal of Ecology,2014,33 (2):510−517. doi: 10.13292/j.1000-4890.2014.0036
[20] 祁栋灵,孙瑞,谢贵水,杨川,陈帮乾,等. 海南西部低割龄橡胶林土壤水分季节变化特征及其对气象因子响应研究初报[J]. 生态科学,2017,36(6):44−48. Qi DL,Sun R,Xie GS,Yang C,Chen BQ,et al. A preliminary study on seasonal changes of soil moisture in rubber plantation of low tapping years and its responses to meteorological factors in western Hainan Island,China[J]. Ecological Science,2017,36 (6):44−48.
[21] Meng S,Ma HB,Li ZS,Yang FC,Wang SK,Lu JK. Impacts of nitrogen on physiological interactions of the hemiparasitic Santalum album and its N2-fxing host Dalbergia odorifera[J]. Trees,2021,35 (3):1039−1051. doi: 10.1007/s00468-021-02103-0
[22] Yao X,Lan Y,Liao L,Huang Y,Yu S,et al. Effects of nitrogen supply rate on photosynthesis,nitrogen uptake and growth of seedlings in a Eucalyptus/Dalbergia odorifera intercropping system[J]. Plant Biol,2022,24 (1):192−204. doi: 10.1111/plb.13341
[23] Xiang LS,Miao LF,Yang F. Drought and nitrogen application modulate the morphological and physiological responses of Dalbergia odorifera to different niche neighbors[J]. Front Plant Sci,2021,12:664122. doi: 10.3389/fpls.2021.664122
[24] 周璋. 氮磷添加对海南热带山地雨林碳循环的影响[D]. 北京: 北京大学, 2013: 1−137. [25] 高俊凤. 植物生理学实验指导[M]. 北京: 高等教育出版社, 2006: 74−77. [26] 崔豫川,张文辉,李志萍. 干旱和复水对栓皮栎幼苗生长和生理特性的影响[J]. 林业科学,2014,50(7):66−73. Cui YC,Zhang WH,Li ZP. Effects of drought stress and rewatering on growth and physiological characteristics of Quercus variabilis seedlings[J]. Scientia Silvae Sinicae,2014,50 (7):66−73.
[27] 王铭涵,丁玎,张晨禹,高羲之,陈建姣,等. 干旱胁迫对茶树幼苗生长及叶绿素荧光特性的影响[J]. 茶叶科学,2020,40(4):478−491. doi: 10.3969/j.issn.1000-369X.2020.04.006 Wang MH,Ding D,Zhang CY,Gao XZ,Chen JJ,et al. Effects of drought stress on growth and chlorophyll fluorescence characteristics of tea seedlings[J]. Journal of Tea Science,2020,40 (4):478−491. doi: 10.3969/j.issn.1000-369X.2020.04.006
[28] Xu NN,Guo WH,Liu J,Du N,Wang RQ. Increased nitrogen deposition alleviated the adverse effects of drought stress on Quercus variabilis and Quercus mongolica seedlings[J]. Acta Physiol Plant,2015,37 (6):107. doi: 10.1007/s11738-015-1853-4
[29] Zhou XB,Zhang YM,Ji XH,Downing A,Serpe M. Combined effects of nitrogen deposition and water stress on growth and physiological responses of two annual desert plants in northwestern China[J]. Environ Exp Bot,2011,74:1−8. doi: 10.1016/j.envexpbot.2010.12.005
[30] 姚春娟,郭圣茂,马英超,赖晓莲,杨肖华. 干旱胁迫对4种决明属植物光合作用和叶绿素荧光特性的影响[J]. 草业科学,2017,34(9):1880−1888. Yao CJ,Guo SM,Ma YC,Lai XL,Yang XH. Effect of drought stress on characteristics of photosynthesis and chlorophyll fluorescence of four species of Cassia[J]. Pratacultural Science,2017,34 (9):1880−1888.
[31] 李泽,谭晓风,卢锟,张琳,龙洪旭,等. 干旱胁迫对两种油桐幼苗生长、气体交换及叶绿素荧光参数的影响[J]. 生态学报,2017,37(5):1515−1524. Li Z,Tan XF,Lu K,Zhang L,Long HX,et al. Influence of drought stress on the growth,leaf gas exchange,and chlorophyll fluorescence in two varieties of tung tree seedlings[J]. Acta Ecologica Sinica,2017,37 (5):1515−1524.
[32] 吴敏,邓平,赵英,赵仕花,陈金妮,等. 喀斯特干旱环境对青冈栎叶片生长及叶绿素荧光动力学参数的影响[J]. 应用生态学报,2019,30(12):4071−4081. doi: 10.13287/j.1001-9332.201912.001 Wu M,Deng P,Zhao Y,Zhao SH,Chen JN,et al. Effects of drought on leaf growth and chlorophyll fluorescence kinetics parameters in Cyclobalanopsis glauca seedlings of Karst areas[J]. Chinese Journal of Applied Ecology,2019,30 (12):4071−4081. doi: 10.13287/j.1001-9332.201912.001
[33] 钟小莉,马晓东,吕豪豪,朱成刚,杨余辉. 干旱胁迫下氮素对胡杨幼苗生长及光合的影响[J]. 生态学杂志,2017,36(10):2777−2786. doi: 10.13292/j.1000-4890.201710.029 Zhong XL,Ma XD,Lü HH,Zhu CG,Yang YH. Effect of nitrogen on growth and photosynthesis of Populus euphratica seedlings under drought stress[J]. Chinese Journal of Ecology,2017,36 (10):2777−2786. doi: 10.13292/j.1000-4890.201710.029
[34] 李志元,江虹,王亚楠,秦亚楠,余婷,等. 施氮与水分胁迫对雪菊幼苗生长及生理的影响[J]. 新疆农业科学,2020,57(1):127−138. Li ZY,Jiang H,Wang YN,Qin YN,Yu T,et al. Effects of water stress and nitrogen application on growth and physiology of Coreopsis tinctoria seedlings[J]. Xinjiang Agricultural Sciences,2020,57 (1):127−138.
[35] Souza BD,Meiado MV,Rodrigues BM,Santos MG. Water relations and chlorophyll fluorescence responses of two leguminous trees from the Caatinga to different watering regimes[J]. Acta Physiol Plant,2010,32 (2):235−244. doi: 10.1007/s11738-009-0394-0
[36] 杨曾奖,徐大平,陈文平,黄烈健,李尚均,陈源. 华南地区桉树/相思混交种植的林木生长效应[J]. 应用生态学报,2009,20(10):2339−2344. doi: 10.13287/j.1001-9332.2009.0338 Yang CJ,Xu DP,Chen WP,Huang LJ,Li SJ,Chen Y. Growth effect of eucalyptus-acacia mixed plantation in South China[J]. Chinese Journal of Applied Ecology,2009,20 (10):2339−2344. doi: 10.13287/j.1001-9332.2009.0338
[37] 许峻模,潘婷,龙佳峰,汤文艳,田诗韵,叶绍明. 施氮及不同根系分隔模式对尾叶桉和降香黄檀幼苗生长及叶片生理特性的影响[J]. 西北植物学报,2018,38(6):1128−1137. doi: 10.7606/j.issn.1000-4025.2018.06.1128 Xu JM,Pan T,Long JF,Tang WY,Tian SY,Ye SM. Effect of nitrogen application on the growth and leaf physiological traits of Eucalyptus urophylla and Dalbergia odorifera seedlings under different root partitioning patterns[J]. Acta Botanica Boreali-Occidentalia Sinica,2018,38 (6):1128−1137. doi: 10.7606/j.issn.1000-4025.2018.06.1128




 下载:
下载: