Impact of leaf absorption of atmospheric heavy metals on plants: a review
-
摘要:
化石燃料燃烧、汽车尾气排放、冶金工业排放、矿山开采等人类活动向大气排放了大量重金属,导致重金属污染日趋严重,严重威胁土壤、水体环境质量和植物安全。大气中的重金属经干湿沉降后,将参与植物系统的生物化学循环。与根系相比,人们对植物叶片吸收重金属的机制知之甚少。本文对前人相关研究结果进行了整理,系统综述了植物叶片吸收、转运大气重金属的机制。植物叶面主要通过气孔和角质层吸收重金属,随后利用质外体或共质体途径转运到韧皮部,最终以与光合产物运输相同的方式向叶、果实、根等器官转运;重金属的形态变化与吸收转运过程有关;毒性机制主要涉及机械损伤、光合相关过程抑制和氧化应激;植物的解毒机制包括植物细胞壁中的滞留、植物可溶性部分中的分隔以及抗氧化系统的防御等。
Abstract:Fossil fuel combustion, vehicle exhaust emissions, metallurgical industry emissions, and mining activities discharge significant amounts of heavy metals into the atmosphere. This has intensified heavy metal pollution, posing serious threats to the environmental quality of soil, water, and plants. After dry and wet deposition from the atmosphere, these heavy metals participate in plant biochemical cycles. While root-based heavy metal uptake is well-documented, the mechanisms and impact of heavy metal absorption by leaves remain poorly studied. This paper summarizes previous work, offering a systematic review of the mechanisms governing plant leaf absorption and transport of atmospheric heavy metals. Plant leaves primarily absorb heavy metals through their stomata and cuticles, with subsequent transport to the phloem through the apoplast or symplast pathway, and final deposition in the leaves, fruits, roots, and other organs, similar to photosynthetic products. Morphological changes in heavy metals in the plant are associated with absorption and transport processes. Toxicity mechanisms primarily involve mechanical damage, inhibition of photosynthesis-related processes, and oxidative stress. Detoxification mechanisms in plants include retention within the cell walls, sequestration in the soluble parts of plants, and defense by the antioxidant system.
-
Keywords:
- Plant leaves /
- Atmospheric heavy metals /
- Toxicity /
- Detoxify
-
近年来,我国工业化进程的加速和城市城镇的快速扩张,导致大气重金属排放的持续增加,其中12种典型大气重金属排放总量从2000年的58 852.5吨增长到2012年的79 570 t[1]。大气重金属在时间和空间上存在显著差异,在2000-2018年间,大气镉(Cd)沉降通量在城市区和农业区逐渐增加,重金属总沉降通量在空间上表现为工业区 > 城市区 > 农业区[2]。化石燃料燃烧、汽车尾气排放、冶金工业排放、矿山开采等人类活动是大气重金属的主要来源[3]。释放到大气中的重金属经过一系列物理化学过程,最终以干湿沉降的方式返回到土壤、水体和植被[4-6]。相较于前两者,植被高大的树冠和更加庞大的叶面接触面积可以对大气沉降重金属先行拦截,并通过叶表面的凹槽、褶皱、突起、绒毛、蜡质等微观结构的协同作用对重金属进行滞留和固定,实现大气重金属的有效沉积[7, 8]。
通过叶面吸收积累的重金属不仅危害植物本身的生理功能,还对人类健康产生潜在的不利影响。Schreck等[9]报道,黑麦草(Lolium perenne L.)叶片通过气孔吸收大气沉降的铅(Pb)破坏了气孔周围的保卫细胞。Kovácik等[10]指出,对空气凤梨(Tillandsia)叶面施加镍(Ni)和Cd会导致其含水量显著降低,进而使糖分、叶绿素a、叶绿素b和类胡萝卜素减少。Xiong等[11]通过氧化铜纳米颗粒(CuO-NPs)的叶面吸收实验发现,在高浓度(250 mg/株)CuO-NPs暴露条件下,卷心菜(Brassica oleracea var. capitata L.)含水量在15天内损失超过50%,同时还伴有一定程度的叶片黄化和坏死现象,且暴露组铜(Cu)日摄入量(EDI)是Cu每日耐受摄入量(TDI)的2~45倍,食用该类蔬菜将严重威胁人类健康。Natasha等[12]评估了食用含砷(As)、Pb叶面吸收累积的菠菜(Spinacia oleracea L.)可能造成的健康风险,结果表明,在所有As、Pb叶面施用水平(50、100 mg/L)下生长的菠菜都能对成人和儿童产生致癌效应。鉴于叶面吸收重金属的重要性,以及重金属污染植物对人类健康的潜在威胁,研究大气重金属的沉降、植物叶片吸收和转运重金属的途径、过程和机制显得尤为重要。
1. 植物叶片吸收重金属的机制及其影响因素
1.1 植物吸收累积重金属的主要途径
土壤重金属被认为是植物积累重金属的主要来源,研究者针对植物对土壤重金属的吸收、转移机制,毒性及解毒机制等开展了大量工作[13-15]。但随着大气污染多源化和污染物种多样化,大气沉降已成为植物重金属积累的主要来源[16, 17]。植物主要通过根系吸收和叶面吸收两种途径积累重金属[18, 19],且叶面吸收对植物重金属积累的贡献比根系吸收更大,是植物重金属积累的关键途径。两种吸收途径对植物重金属积累的贡献度见表1。
表 1 植物吸收重金属途径的贡献度Table 1. Contribution degree of heavy metal uptake by plants重金属
Heavy metal实验条件
Experimental condition植物种类
Plant species重金属吸收量
Heavy metal absorption参考
文献
ReferenceCu 田间实验:自然进行叶面和根系暴露
温室实验:人工设置叶面和根系暴露小白菜
Brassica chinensis L.田间和温室条件下,叶片吸收的Cu占比分别为50%~84%和90%~96%。 [30] Ag 温室实验:人工设置叶面和根系暴露 大豆Glycine max (L.) Merr.
水稻Oryza sativa L.类似施用水平下,叶面暴露积累的Ag是根系暴露的17~200倍。 [31] Cd 田间实验:自然进行叶面和根系暴露
温室实验:人工设置叶面和根系暴露小白菜
Brassica chinensis L.田间和温室条件下,叶片吸收的Cd占比分别为38%~88%和64%~80%。 [30] 田间实验:自然进行叶面和根系暴露
温室实验:人工设置叶面和根系暴露小白菜
Brassica chinensis L.田间和温室条件下,叶片吸收的Pb占比分别为70%~99%和92%~95%。 [30] Pb 田间实验:自然进行叶面和根系暴露 小麦
Triticum aestivum L.叶片吸收的Pb占总含量的82.48%~92.79%。 [32] 温室实验:人工设置叶面和根系暴露 茄子
Solanum melongena L.叶面吸收的Pb是根系吸收的25.1~44.1倍。 [33] 1.2 植物叶片吸收重金属的机制
大气中的重金属能够以气态或颗粒态的形式干沉降到植物叶表面[20],也可以通过溶解态的湿沉降形式沉积在植物叶表面[4-6]。沉积在叶表面的重金属90%以上会在强降水和风力作用下回到环境中,剩余的则会吸附在叶表面被植物体吸收[21, 22]。重金属在植物叶面上的吸附是通过物理吸附和生化吸附两种机制进行的[23],物理吸附与植物叶片的物理特性有关,尤其是叶面特征[24]。Li等[25]研究表明,叶面的凹槽、绒毛和气孔可以增加叶面的粗糙度,从而保留更多的颗粒。Sun等[26]指出,具有粗糙表面、高气孔密度、致密绒毛和其他微观结构的植物叶面可以保留更多的颗粒物质。总体而言,复杂多样的微观结构和粗糙的叶面意味着更多的颗粒可以接触叶面,产生更强大的保留能力[27]。生化吸附与植物叶面的蜡质、树脂、黏液等分泌物有关。一般而言,分泌物越多,吸附的颗粒物越多[28, 29]。与物理吸附相比,生化吸附保留颗粒物的能力更强。这是因为植物叶面分泌物对大气中的颗粒物有较强的黏附作用,可以快速地吸附颗粒物,且不易受外界环境的变化而轻易脱落[29, 34, 35]。
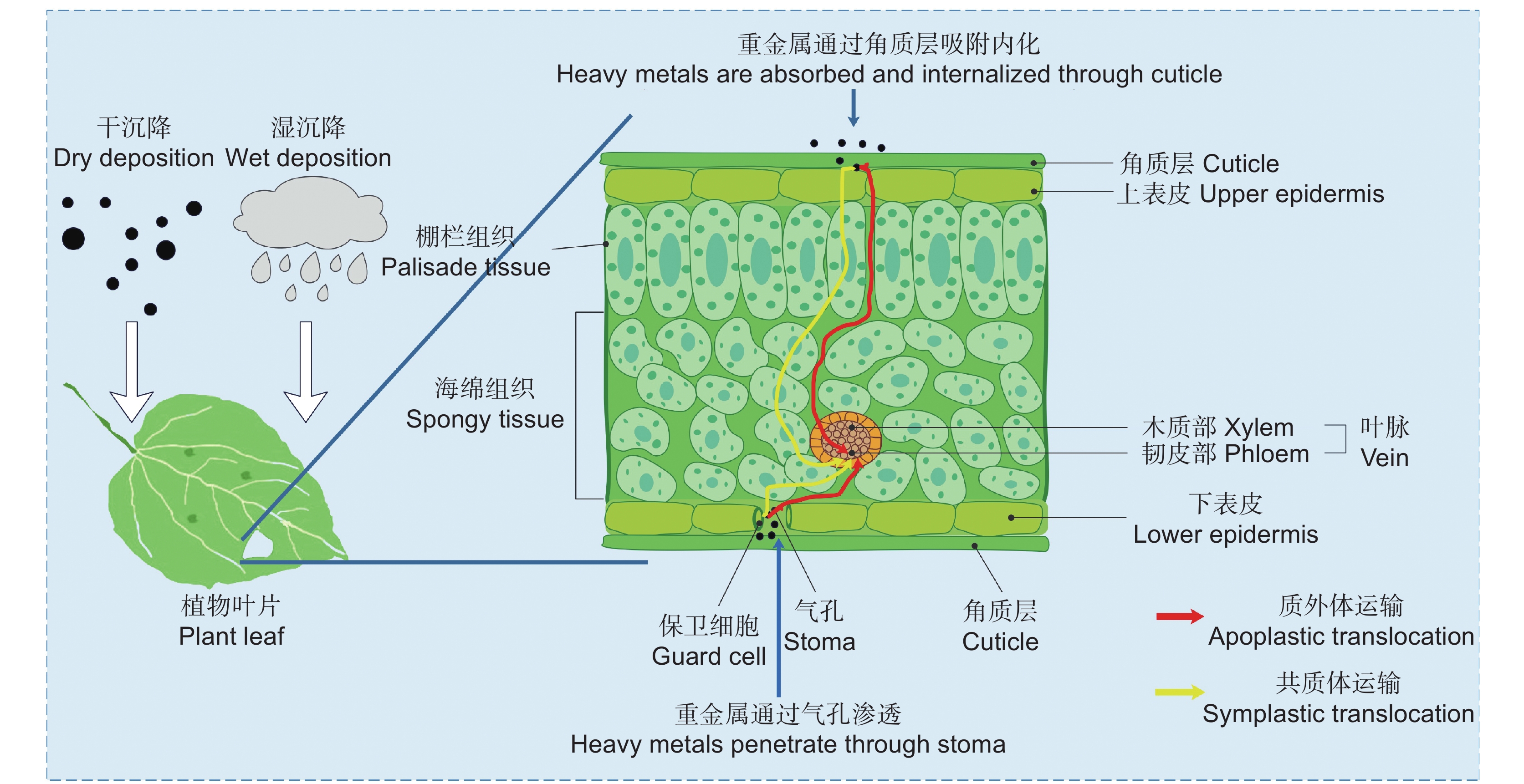
植物叶面重金属吸收主要通过气孔吸收和角质层吸附内化两种机制进行[36-38](图1)。气孔吸收是指叶面沉积颗粒覆盖了叶表皮的气孔开口及其保卫细胞,导致气孔无法关闭而保持永久开放,其中部分携带重金属的细小颗粒物通过开放气孔进入叶组织。除了开放气孔外,在气孔中还存在细小的孔隙,与颗粒物结合的重金属可以通过这些孔隙渗透到叶组织内部[39, 40]。角质层吸附内化是指叶面沉积的重金属颗粒物吸附到表皮蜡中,浸入蜡质层,被蜡质包覆的重金属通过内吞作用穿透角质层,进入角化层,随后在质外体中解吸,通过果胶等物质进入表皮细胞[18, 36]。Chamel等[41]将角质层吸附内化过程分为4个步骤:(1)粘附在表皮上;(2)穿透角质层;(3)质外体中的解吸;(4)下层细胞的吸收。根据重金属疏水性的差异,角质层有两条独特的吸收通道:亲脂性通道和亲水性通道[36, 42]。亲脂性通道是由蜡质和角质沉积而成的固有通道[43]。亲水性通道是临时通道,其形成与角质层内的永久性偶极子和离子官能团的水化作用有关[44]。一般来说,重金属颗粒物的亲脂亲水性是确定的,但重金属颗粒物的亲脂亲水性可能在叶面吸收过程中发生改变。如,裸露的银纳米离子是亲水的,但在吸收过程中可能被表皮蜡包覆,这可能会增加它的亲脂性,使其通过亲脂性通道被吸收[42]。
1.3 植物叶片吸收重金属的影响因素
植物叶片对重金属的吸收受到多种因素的影响,包括叶面特征、颗粒物的尺寸、环境因素等。
植物叶片吸收重金属的多少与吸附在叶面的重金属多少有关。叶面吸附重金属的能力与叶面特征及叶面分泌物有关。研究表明,叶面粗糙、具有复杂微观结构(有绒毛、褶皱、凹槽等)、气孔密度高、叶面积大、含有丰富分泌物(蜡质、树脂、黏液等)的植物叶片可以保留更多的颗粒物,从而具有吸收更多重金属的可能[25-29]。
颗粒物的尺寸是限制叶面吸收重金属的主要因素。气孔和角质层存在尺寸限制,气孔孔径大,可以吸收直径在几微米及以下的重金属,角质层孔隙较小,只能针对几纳米的微粒[42, 45, 46]。叶面暴露后,Ouyang等[47]使用扫描电子显微镜(SEM)和X-射线能量色散谱仪(EDS)分析了两种蔬菜叶片中的CdS分布情况,发现其以微米聚集体的形式存在于气孔内部,而在表皮蜡和角质层内部未发现任何形式的CdS。Chen等[48]使用微米级金刚石粉作为示踪剂来模拟PM2.5在植物叶片上的吸收,结果发现金刚石颗粒仅存在于叶片气孔区。有研究者通过叶面暴露实验,发现小于100 nm的颗粒可以穿过角质层,进入叶片内部[49, 50]。因此,当沉降颗粒达到微米级时,气孔是植物吸收重金属的主要途径,当沉降颗粒小于100 nm时,气孔和角质层共同吸收重金属。
湿度、温度、降水等是影响植物叶片吸收重金属的主要环境因素。低湿和高温环境会导致气孔关闭和角质层收缩,进而抑制植物叶片对重金属的吸收[51]。高湿环境有助于增强叶面的水化作用,进而提高叶面渗透势,使重金属在叶片表面易于渗透[52, 53]。降水影响颗粒物的沉积,降水量越大,持续时间越长,沉积颗粒去除的越多,叶片吸收的重金属则越少[54]。
2. 重金属被叶片吸收后在植物体内的转运
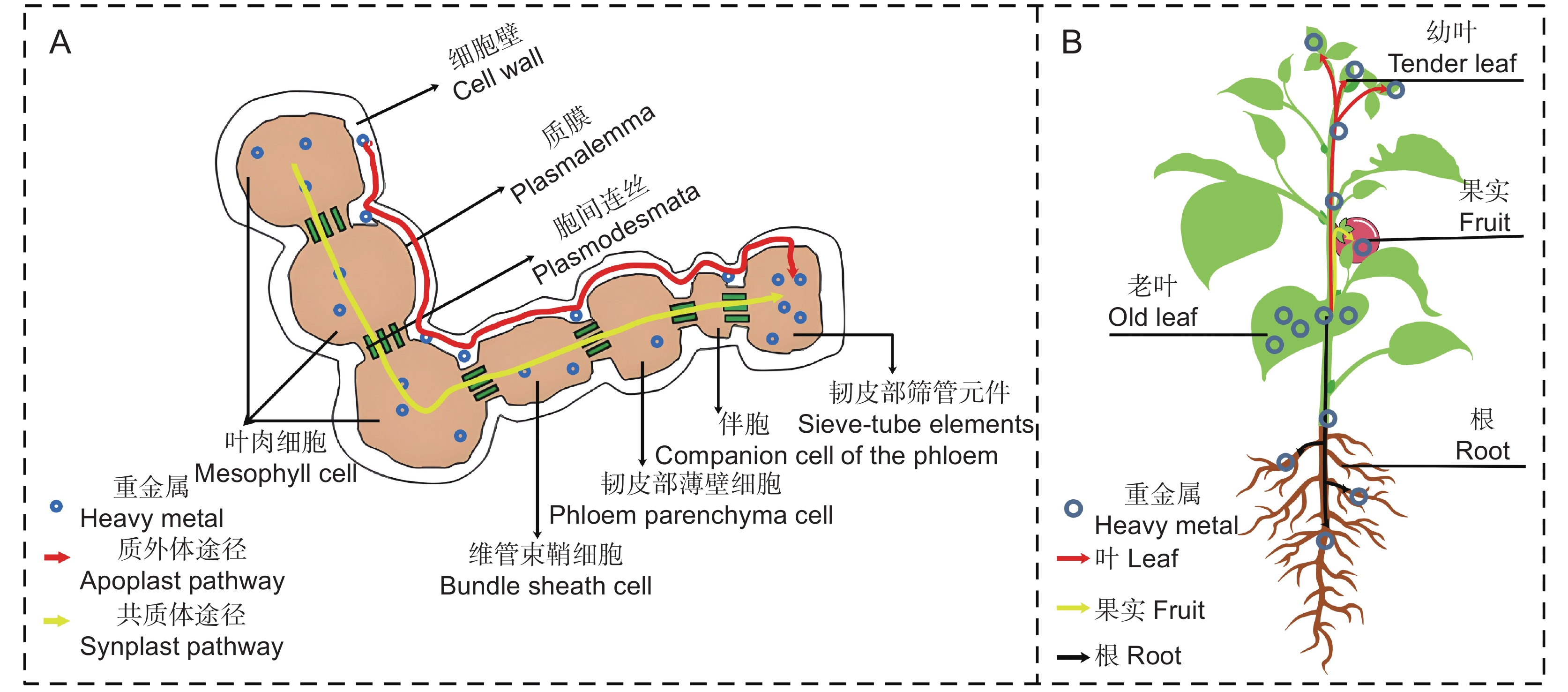
重金属穿透叶表皮后,必须再穿过几个屏障才能到达韧皮部。首先,穿透叶表皮的重金属沉积在细胞壁上,之后,一部分重金属进入细胞间隙与质外体结合,一部分穿过细胞壁进入共质体[47] (图1)。重金属通过质外体或共质体途径移动到与伴胞相连的维管束鞘细胞,进而进入韧皮部的筛管元件[55] (图2:A),最终向其他器官转运。韧皮部引导的重金属转运过程与光合产物的运输过程类似,都是通过韧皮部维管系统将目标转运物运输到植物的其他器官[56, 57]。重金属主要被转运到叶、果实和根中[55](图2:B)。
植物倾向于将重金属从成熟叶转运到发育中的叶片。重金属在两种叶中的转运可能与植物的自我保护和重金属耐性机制有关。Hu等[58]发现,富含Cd的超积累植物对蚕豆蚜的侵染具有更强的抵抗力,这是因为蚕豆蚜在吞食富含Cd的幼嫩植物后会呈现中毒现象,进而诱导其放弃吞食。因此, Cd可能在幼嫩韧皮部中起着防御化合物的作用,防止虫类的吞食,形成植物自我保护策略的一个组成部分。既使是对重金属有很高耐受性的超积累植物,也会因为过量积累重金属而引起严重的毒害效应[59],因此,韧皮部运输介导的Cd重新分配也可能是一种金属耐受机制,以避免Cd在叶和茎中过度积累最终导致严重的毒害[58]。
叶面吸收的重金属向果实的转运与果实的发育有关,果实在发育过程中需要吸收来自叶片光合作用产生的养分,重金属则通过韧皮部同养分一起向果实转运[60]。叶面吸收的重金属向果实的转运只能在果实发育期进行。Ma等[61]研究表明,灌浆期是小麦(Triticum aestivum L.)叶片Cd向籽粒转运的主要时期,小麦籽粒中的Cd有两个来源,一是小麦开花期叶片吸收储存的Cd在灌浆期的再分配,二是灌浆期叶面新吸收Cd的韧皮部运输,其中灌浆前期是叶片Cd向小麦籽粒转运的关键时期。叶片重金属的转运是果实重金属的主要来源,Ma等[60]研究发现,叶对小麦籽粒Pb的相对贡献率为32.58%,是除穗之外的第二大来源。Xu等[62]报道了Cd从水稻(Oryza sativa L.)叶向籽粒转运的特征,转移因子(TF)在0.18~0.28,尽管转运量低,但转运的Cd浓度均超过了中国国家标准的最大允许浓度(MPC)(0.2 mg/kg)。
叶面吸收的重金属除了在植物地上部进行转运外,还会向根部迁移[63]。Dollard[64]报道,萝卜(Raphanus sativus L.)叶面施用的Pb可以向根部转运,转运的量小于施用量的0.3%。Xiong等[65]研究表明,莴苣(Lactuca sativa L.)叶和卷心菜中,PbO颗粒可以从叶转移到根, TF范围仅在0.06~0.21。Deng等[66]发现,遏蓝菜(Thlaspi arvense L.)叶面吸收的镍有11%向下转移到根部。叶面吸收的重金属可以向根部转移,但转移的量较少,这可能与大量重金属与叶细胞壁或与叶细胞内的植物螯合素结合有关[67]。
重金属在韧皮部的转运并不是必需的。金属离子与细胞壁上带负电荷基团发生位点的结合可以限制它们在植物叶片内的运动[18, 19]。特定细胞的区隔也可以限制重金属的转运,当细胞壁结合位点达到饱和时,重金属可以通过细胞膜进入细胞和液泡,然后储存在可溶性部分(主要是液泡)抑制其转运[68]。
3. 叶片吸收与转运对重金属形态的影响
沉降重金属在植物体上的行为会引发重金属形态的变化,这种变化可能与叶面吸收与转运过程有关[9],几种假设如下:
(1) 叶面吸收过程中,湿度改变可能使叶表面的颗粒物发生变化[69]。有研究发现,空气湿度小于100%时,FeⅢ螯合物以固态形式吸附在杨(Populus)叶表面,而不被叶片吸收;当空气湿度达到100%时,FeⅢ螯合物溶解成液态,进而被叶片吸收[70]。Schreck等[9]通过拉曼显微光谱(RMS)在生菜(Lactuca sativa var. ramosa Hort.)叶片表面鉴定出了六方片状PbCO3晶体,该晶体的形成与Pb + 和CO32-在潮湿大气中的增溶-结晶过程有关。
(2) 重金属的形态变化可能是其在转运过程中与叶片中的化合物(富硫肽、有机酸、草酸盐、多糖等)相结合的结果[71]。Schreck等[9]证明了转运过程中无机态重金属向有机态的转化,他们在黑麦草叶片中观察到大量有机Pb,这些有机Pb是在转运过程中与细胞壁多糖和叶片有机酸相结合所形成的。
(3) 植物叶片表面通常被多种微生物定居,微生物之间的相互作用可能引起重金属的形态变化[72, 73]。微生物对重金属形态的影响涉及根暴露实验[74],目前尚没有叶面微生物影响重金属形态的相关报道,该假设尚未被证实。
4. 植物叶面吸收重金属后的毒性与解毒
4.1 植物叶面吸收重金属后的毒性
叶面吸收的重金属可引发植物一系列生理和代谢变化,这些变化与重金属毒性和植物解毒效应有关[75]。植株矮小、生长缓慢、叶片失绿黄化、叶片失水干枯等是重金属对植物产生毒性的外在表现[11, 65, 76],其内在毒性机制复杂,主要表现为促进机械损伤、抑制光合相关过程、诱导氧化应激等[77, 78]。
重金属和细胞的相互作用可能会对细胞结构造成机械损伤,如堵塞导管、细胞壁孔和气孔[11, 79];使气孔长期开放等[9]。导管、细胞壁孔和气孔的堵塞将阻碍营养的吸收和运输,影响植物与外界的气体交换和水分蒸发,从而影响蒸腾、光合、呼吸等作用。气孔的长期开放会导致重金属的持续摄入,造成更严重的毒害。
重金属抑制植物光合作用的相关过程。叶绿素是植物进行光合作用的主要色素,其生物合成过程包括数十步酶促反应[80]。在酶促反应中,重金属与叶绿素生物合成酶-SH基团的结合可以抑制叶绿素的合成[81],进而影响光合作用。Lu等[82]报道,叶片中的氧化铁纳米颗粒能够触发类芬顿反应,并产生过量的羟基自由基(·OH),过量的·OH能够加速叶绿素的降解,导致叶绿素含量和光合作用的降低。研究表明,一定浓度的Cu在叶片中显现毒性,抑制光合活性[11, 83],这种光合活性的抑制可能是光系统Ⅱ反应中心的失活、电子传输的减少以及热能耗散增加的结果[84]。总之,叶面暴露重金属后,植物光合相关过程的抑制主要涉及叶绿素的合成抑制和加速降解、光合活性降低、光合作用单位破坏等方面。
活性氧(ROS)是植物体有氧代谢的必然产物,主要包括过氧化氢(H2O2)、单线态氧(1O2)、超氧阴离子自由基(O2∙−)、·OH等含氧分子和含氧自由基[85, 86]。在重金属暴露条件下,植物可以通过叶绿体、线粒体、过氧化物酶体、质外体、内质网、细胞壁和质膜等多种细胞区室产生ROS[86, 87]。当ROS超出其清除系统的阈值时,就会产生氧化应激,破坏脂质、蛋白质、核酸等生物大分子,最终导致细胞死亡[85-87]。ROS造成的氧化应激的主要目标是植物细胞中的脂质和蛋白质[85]。脂质由多不饱和脂肪酸(PUFA)组成,PUFA在ROS作用下的氧化分解称为脂质过氧化[87]。过量的脂质过氧化会损害膜蛋白,增加细胞膜的流动性和通透性,最终改变细胞的结构和功能[86, 88]。ROS攻击蛋白质,从而导致蛋白质的亚硝基化、羰基化、谷硫磺酰化、以及形成二硫键等修饰[86],并最终导致蛋白质活性降低、结构改变、功能失常和降解[86, 88]。
4.2 叶面吸收重金属后植物的解毒机制
植物通过多种机制对抗重金属的毒性,包括植物细胞壁中的滞留、植物可溶性部分中的分隔以及抗氧化系统的防御等[89, 90]。
植物细胞壁的滞留和可溶性部分中的分隔是有效的重金属解毒机制,其内在机理是限制重金属的转运,避免重金属在植物组织或器官中的过度积累产生毒害作用。细胞壁是保护原生质体免受重金属毒害的第一道屏障[91],其通过负电荷基团与重金属结合,从而限制后者的转运[19]。然而,细胞壁上的重金属结合位点是有限的,当其达到饱和时,重金属穿透细胞壁进入并储存在可溶性部分(主要是液泡)和其他细胞器中[68]。经过细胞壁和可溶性部分的拦截,进入其他细胞器的重金属显著减少,不足以造成毒害。Ouyang等[47]报道,在大气Cd沉降下,空心菜(Ipomoea aquatica Forssk)和白菜(Brassica rapa var. glabra Regel )没有表现出明显的毒性症状,这可能与细胞壁和可溶性部分限制Cd的转运有关,因为叶片吸收的Cd主要存在于细胞壁和可溶性部分。
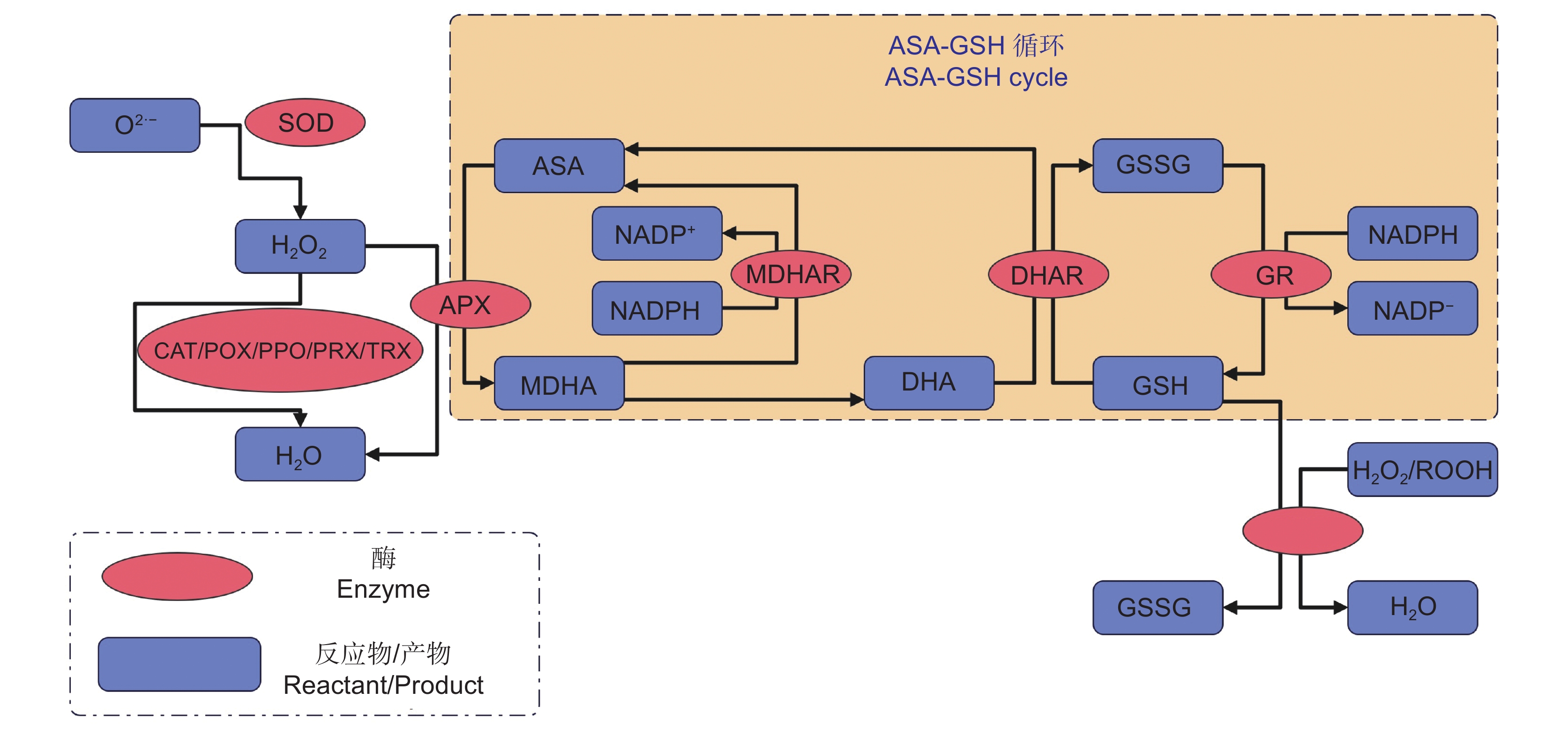
为了避免ROS引起的氧化损伤,植物会提高自身的抗氧化能力,清除多余的ROS。参与ROS清除的抗氧化防御系统由抗氧化酶和非酶抗氧化剂组成。抗氧化酶包括超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、抗坏血酸过氧化物酶(APX)、单脱氢抗坏血酸还原酶(MDHAR)、脱氢抗坏血酸还原酶(DHAR)、谷胱甘肽还原酶(GR)、谷胱甘肽过氧化物酶(GPX)和其他几种酶[92, 93]。非酶抗氧化剂由抗坏血酸(AsA)、谷胱甘肽(GSH)和少数其他抗氧化剂组成[92, 93]。其中APX、MDHAR、DHAR、GR、AsA和GSH构成了AsA-GSH 循环。抗氧化酶存在于不同的细胞器中,在氧化应激时协同响应,清除过量的ROS。如,SOD先将O2∙−歧化为H2O2,之后,H2O2在CAT、APX、GPX等多种酶的作用下还原成H2O[92](图3)。非酶抗氧化剂是一类重要的ROS清除剂,其不仅作为自由基清除剂直接发挥作用,还作为辅助因子参与抗氧化过程[94]。AsA-GSH 循环在抗氧化防御中起着关键作用,其中,APX、MDHAR、DHAR、GR 等4种酶保证AsA-GSH循环的持续运行,AsA和GSH为抗氧化反应提供电子,直接清除ROS,使氧化应激最小化[94, 95] (图3)。
5. 展望
大气重金属以干湿沉降的方式沉积于植物叶面,主要通过气孔吸收和表皮角质层吸附内化进入叶片内部。进入叶片内部的重金属先通过质外体或共质体向韧皮部转运,再从韧皮部向叶、果实和根等器官转运。在吸收和转运过程中伴随着重金属的形态变化,这种变化与湿度以及植物体内的化合物有关。进入植物体内的重金属会对植物产生毒性,主要是诱导氧化应激,进而破坏脂质、蛋白质、核酸等生物大分子,最终导致细胞死亡。抗氧化防御系统是植物应对氧化应激的解毒机制,主要通过抗氧化酶和非酶抗氧化剂清除过量的ROS,从而降低氧化应激程度。在大气沉降重金属增加的背景下,叶片对大气重金属的吸收、转运和影响越发重要,虽然目前已经取得了一定的研究进展,但仍存在如下科学问题亟待解决:(1)叶面与根系吸收的重金属毒性和解毒机制是否存在区别;(2)叶面微生物对植物叶片吸收重金属的影响如何;(3)叶面吸收的重金属如何在植物各器官间分配及其定量;(4)敏感型、耐受型和超耐受型植物解毒机制的差异等。这些问题的解决可促进我们对重金属在大气和植物界面生物化学行为的深入认识,为生态环境和人类健康的可持续发展提供理论基础。
-
表 1 植物吸收重金属途径的贡献度
Table 1 Contribution degree of heavy metal uptake by plants
重金属
Heavy metal实验条件
Experimental condition植物种类
Plant species重金属吸收量
Heavy metal absorption参考
文献
ReferenceCu 田间实验:自然进行叶面和根系暴露
温室实验:人工设置叶面和根系暴露小白菜
Brassica chinensis L.田间和温室条件下,叶片吸收的Cu占比分别为50%~84%和90%~96%。 [30] Ag 温室实验:人工设置叶面和根系暴露 大豆Glycine max (L.) Merr.
水稻Oryza sativa L.类似施用水平下,叶面暴露积累的Ag是根系暴露的17~200倍。 [31] Cd 田间实验:自然进行叶面和根系暴露
温室实验:人工设置叶面和根系暴露小白菜
Brassica chinensis L.田间和温室条件下,叶片吸收的Cd占比分别为38%~88%和64%~80%。 [30] 田间实验:自然进行叶面和根系暴露
温室实验:人工设置叶面和根系暴露小白菜
Brassica chinensis L.田间和温室条件下,叶片吸收的Pb占比分别为70%~99%和92%~95%。 [30] Pb 田间实验:自然进行叶面和根系暴露 小麦
Triticum aestivum L.叶片吸收的Pb占总含量的82.48%~92.79%。 [32] 温室实验:人工设置叶面和根系暴露 茄子
Solanum melongena L.叶面吸收的Pb是根系吸收的25.1~44.1倍。 [33] -
[1] Tian HZ,Zhu CY,Gao JJ,Cheng K,Hao JM,et al. Quantitative assessment of atmospheric emissions of toxic heavy metals from anthropogenic sources in China:historical trend,spatial distribution,uncertainties,and control policies[J]. Atmos Chem Phys,2015,15 (17):10127−10147. doi: 10.5194/acp-15-10127-2015
[2] 陈其永,郜允兵,倪润祥,潘瑜春,阎跃观,等. 2000-2018 年我国大气重金属沉降通量时空变化特征[J]. 环境科学,2022,43(9):4413−4424. Chen QY,Gao YB,Ni RX,Pan YC,Yan YG,et al. Temporal and spatial variation characteristics of heavy metal in atmospheric deposition in China from 2000 to 2018[J]. Environmental Science,2022,43 (9):4413−4424.
[3] 王鑫,刘冬跃,钱松山,王新,崔文超,马宁. 大气沉降对环境污染研究现状及进展[J]. 北京水务,2021(2):16−20. doi: 10.19671/j.1673-4637.2021.02.004 Wang X,Liu DY,Qian SS,Wang X,Cui WC,Ma N. Research progress of atmospheric deposition on environmental pollution[J]. Beijing Water,2021 (2):16−20. doi: 10.19671/j.1673-4637.2021.02.004
[4] Guo LC,Bao LJ,She JW,Zeng EY. Significance of wet deposition to removal of atmospheric particulate matter and polycyclic aromatic hydrocarbons:a case study in Guangzhou,China[J]. Atmos Environ,2014,83:136−144. doi: 10.1016/j.atmosenv.2013.11.012
[5] Liu JK,Zhu LJ,Wang HH,Yang YL,Liu JT,et al. Dry deposition of particulate matter at an urban forest,wetland and lake surface in Beijing[J]. Atmospheric Environ,2016,125:178−187. doi: 10.1016/j.atmosenv.2015.11.023
[6] Zhu JX,Wang QF,Yu HL,Li ML,He NP. Heavy metal deposition through rainfall in Chinese natural terrestrial ecosystems:evidences from national-scale network monitoring[J]. Chemosphere,2016,164:128−133. doi: 10.1016/j.chemosphere.2016.08.105
[7] Mcdonald AG,Bealey WJ,Fowler D,Dragosits U,Skiba U,et al. Quantifying the effect of urban tree planting on concentrations and depositions of PM10 in two UK conurbations[J]. Atmos Environ,2007,41 (38):8455−8467. doi: 10.1016/j.atmosenv.2007.07.025
[8] Shao F,Wang LH,Sun FB,Li G,Yu L,et al. Study on different particulate matter retention capacities of the leaf surfaces of eight common garden plants in Hangzhou,China[J]. Sci Total Environ,2019,652:939−951. doi: 10.1016/j.scitotenv.2018.10.182
[9] Schreck E,Dappe V,Sarret G,Sobanska S,Nowak D,et al. Foliar or root exposures to smelter particles:consequences for lead compartmentalization and speciation in plant leaves[J]. Sci Total Environ,2014,476-477:667−676. doi: 10.1016/j.scitotenv.2013.12.089
[10] Kováčik J,Klejdus B,Štork F,Hedbavny J. Physiological responses of Tillandsia albida (Bromeliaceae) to long-term foliar metal application[J]. J Hazardous Mater,2012,239-240:175−182. doi: 10.1016/j.jhazmat.2012.08.062
[11] Xiong TT,Dumat C,Dappe V,Vezin H,Schreck E,et al. Copper oxide nanoparticle foliar uptake,phytotoxicity,and consequences for sustainable urban agriculture[J]. Environ Sci Technol,2017,51 (9):5242−5251. doi: 10.1021/acs.est.6b05546
[12] Natasha,Shahid M,Khalid S. Foliar application of lead and arsenic solutions to Spinacia oleracea:biophysiochemical analysis and risk assessment[J]. Environ Sci Pollut Res Int,2020,27 (32):39763−39773. doi: 10.1007/s11356-019-06519-7
[13] Steingräber LF,Ludolphy C,Metz J,Kierdorf H,Kierdorf U. Uptake of lead and zinc from soil by blackberry plants (Rubus fruticosus L. agg. ) and translocation from roots to leaves[J]. Environ Adv,2022,9:100313. doi: 10.1016/j.envadv.2022.100313
[14] Rahman SU,Nawaz MF,Gul S,Yasin G,Hussain B,et al. State-of-the-art OMICS strategies against toxic effects of heavy metals in plants:a review[J]. Ecotoxicol Environ Saf,2022,242:113952. doi: 10.1016/j.ecoenv.2022.113952
[15] Noor I, Sohail H, Sun JX, Nawaz MA, Li GH, et al. Heavy metal and metalloid toxicity in horticultural plants: tolerance mechanism and remediation strategies[J]. Chemosphere, 2022, 303(Pt 3): 135196.
[16] Feng WL,Guo ZH,Xiao XY,Peng C,Shi L,et al. Atmospheric deposition as a source of cadmium and lead to soil-rice system and associated risk assessment[J]. Ecotoxicol Environ Saf,2019,180:160−167. doi: 10.1016/j.ecoenv.2019.04.090
[17] Liu HL,Zhou J,Li M,Obrist D,Wang XZ,Zhou J. Chemical speciation of trace metals in atmospheric deposition and impacts on soil geochemistry and vegetable bioaccumulation near a large copper smelter in China[J]. J Hazard Mater,2021,413:125346. doi: 10.1016/j.jhazmat.2021.125346
[18] 朱臻,杨相东,徐章倩,费讲驰,彭建伟,等. 农作物叶片对大气沉降重金属的吸收转运和积累机制[J]. 植物营养与肥料学报,2021,27(2):332−345. doi: 10.11674/zwyf.20258 Zhu Z,Yang XD,Xu ZQ,Fei JC,Peng JW,et al. Foliar uptake,translocation and accumulation of heavy metals from atmospheric deposition in crops[J]. Journal of Plant Nutrition and Fertilizers,2021,27 (2):332−345. doi: 10.11674/zwyf.20258
[19] Shahid M,Dumat C,Khalid S,Schreck E,Xiong TT,Niazi NK. Foliar heavy metal uptake,toxicity and detoxification in plants:a comparison of foliar and root metal uptake[J]. J Hazard Mater,2017,325:36−58. doi: 10.1016/j.jhazmat.2016.11.063
[20] De Temmerman L,Waegeneers N,Claeys N,Roekens E. Comparison of concentrations of mercury in ambient air to its accumulation by leafy vegetables:an important step in terrestrial food chain analysis[J]. Environ Pollut,2009,157 (4):1337−1341. doi: 10.1016/j.envpol.2008.11.035
[21] 刘辰明,张志强,陈立欣,邹瑞,张璐,等. 降雨对北方城市5种典型城市绿化树种叶面滞尘的影响[J]. 生态学报,2018,38(7):2353−2361. Liu CM,Zhang ZQ,Chen LX,Zou R,Zhang L,et al. Impacts of rainfall on leaf PM (airborne particulate matter) detention in five commonly used urban tree species in northern China[J]. Acta Ecologica Sinica,2018,38 (7):2353−2361.
[22] 查燕. 气象因子对城市植物叶面颗粒物的影响研究[J]. 安徽农业科学,2015,43(20):246−248. doi: 10.13989/j.cnki.0517-6611.2015.20.088 Zha Y. Research on the effect of meteorological factors on urban plant foliar particulate matter[J]. Journal of Anhui Agricultural Sciences,2015,43 (20):246−248. doi: 10.13989/j.cnki.0517-6611.2015.20.088
[23] Kinnersley RP,Scott LK. Aerial contamination of fruit through wet deposition and particulate dry deposition[J]. J Environ Radioact,2001,52 (2-3):191−213. doi: 10.1016/S0265-931X(00)00033-3
[24] Prajapati SK,Tripathi BD. Seasonal variation of leaf dust accumulation and pigment content in plant species exposed to urban particulates pollution[J]. J Environ Qual,2008,37 (3):865−870. doi: 10.2134/jeq2006.0511
[25] Li XL,Zhang TR,Sun FB,Song XM,Zhang YK,et al. The relationship between particulate matter retention capacity and leaf surface micromorphology of ten tree species in Hangzhou,China[J]. Sci Total Environ,2021,771:144812. doi: 10.1016/j.scitotenv.2020.144812
[26] Sun XD,Li HM,Guo X,Sun YK,Li SM. Capacity of six shrub species to retain atmospheric particulates with different diameters[J]. Environ Sci Pollut Res,2018,25 (3):2643−2650. doi: 10.1007/s11356-017-0549-2
[27] Dang N,Zhang HD,Salam MMA,Li HM,Chen GC. Foliar dust particle retention and metal accumulation of five garden tree species in Hangzhou:Seasonal changes[J]. Environ Pollut,2022,306:119472. doi: 10.1016/j.envpol.2022.119472
[28] Przybysz A,Sæbø A,Hanslin HM,Gawroński SW. Accumulation of particulate matter and trace elements on vegetation as affected by pollution level,rainfall and the passage of time[J]. Sci Total Environ,2014,481:360−369. doi: 10.1016/j.scitotenv.2014.02.072
[29] 刘维欢,李维维,裴顺祥,李晓刚. 我国常见园林植物叶片滞尘能力分析[J]. 林业与生态科学,2021,36(3):328−336. Liu WH,Li WW,Pei SX,Li XG. Analysis on the dust-retention ability of common garden plants in China[J]. Forestry and Ecological Sciences,2021,36 (3):328−336.
[30] Liu HL,Zhou J,Li M,Xia RZ,Wang XZ,Zhou J. Dynamic behaviors of newly deposited atmospheric heavy metals in the soil-pak choi system[J]. Environ Sci Technol,2022,56 (17):12734−12744. doi: 10.1021/acs.est.2c04062
[31] Li CC,Dang F,Li M,Zhu M,Zhong H,et al. Effects of exposure pathways on the accumulation and phytotoxicity of silver nanoparticles in soybean and rice[J]. Nanotoxicology,2017,11 (5):699−709. doi: 10.1080/17435390.2017.1344740
[32] Ma C,Liu FY,Hu B,Wei MB,Zhao JH,et al. Direct evidence of lead contamination in wheat tissues from atmospheric deposition based on atmospheric deposition exposure contrast tests[J]. Ecotoxicol Environ Saf,2019,185:109688. doi: 10.1016/j.ecoenv.2019.109688
[33] 张大庚,栗杰,姚忠丽. 不同形态大气铅沉降对茄苗生长和铅积累特征影响[J]. 农业资源与环境学报,2023,40(3):626−635. doi: 10.13254/j.jare.2022.0439 Zhang DG,Li J,Yao ZL. Effects of Pb deposited from the atmosphere on the growth and Pb accumulation characteristics of eggplant seedlings[J]. Journal of Agricultural Resources and Environment,2023,40 (3):626−635. doi: 10.13254/j.jare.2022.0439
[34] Birbaum K,Brogioli R,Schellenberg M,Martinoia E,Stark WJ,et al. No evidence for cerium dioxide nanoparticle translocation in maize plants[J]. Environ Sci Technol,2010,44 (22):8718−8723. doi: 10.1021/es101685f
[35] 林鑫涛,叶诺楠,王彬,陈健,游诗雪,张昕丽. 亚热带常绿树种对不同粒径颗粒物的滞留能力[J]. 广西植物,2016,36(2):170−176,185. Lin XT,Ye NN,Wang B,Chen J,You SX,Zhang XL. Different sizes of particulate matters deposited on leaf of typical subtropical evergreen species[J]. Guihaia,2016,36 (2):170−176,185.
[36] 卢绘,张银龙,吴永波. 植物叶面吸收污染物机制研究进展[J]. 环境化学,2020,39(12):3371−3383. Lu H,Zhang YL,Wu YB. Research progress on foliar absorption mechanism of pollutants[J]. Environmental Chemistry,2020,39 (12):3371−3383.
[37] Gao PP,Xue PY,Dong JW,Zhang XM,Sun HX,et al. Contribution of PM2.5-Pb in atmospheric fallout to Pb accumulation in Chinese cabbage leaves via stomata[J]. J Hazard Mater,2021,407:124356. doi: 10.1016/j.jhazmat.2020.124356
[38] Shahid M,Natasha,Dumat C,Niazi NK,Xiong TT,et al. Ecotoxicology of heavy metal(loid)-enriched particulate matter:foliar accumulation by plants and health impacts[J]. Rev Environ Contam Toxicol,2021,253:65−113.
[39] Ma C,Xie P,Zhang K,Yang JX,Li XZ,et al. Contribution of the flag leaf to lead absorption in wheat grain at the grain-filling stage[J]. Ecotoxicol Environ Saf,2021,225:112722. doi: 10.1016/j.ecoenv.2021.112722
[40] Gajbhiye T,Pandey SK,Kim KH,Szulejko JE,Prasad S. Airborne foliar transfer of PM bound heavy metals in Cassia siamea:a less common route of heavy metal accumulation[J]. Sci Total Environ,2016,573:123−130. doi: 10.1016/j.scitotenv.2016.08.099
[41] Chamel A,Pineri M,Escoubes M. Quantitative determination of water sorption by plant cuticles[J]. Plant Cell Environ,1991,14 (1):87−95. doi: 10.1111/j.1365-3040.1991.tb01374.x
[42] Larue C,Castillo-Michel H,Sobanska S,Cécillon L,Bureau S,et al. Foliar exposure of the crop Lactuca sativa to silver nanoparticles:evidence for internalization and changes in Ag speciation[J]. J Hazard Mater,2014,264:98−106. doi: 10.1016/j.jhazmat.2013.10.053
[43] Riederer M. Biology of the plant cuticle [M]//Riederer M, Müller C, eds. Annual Plant Reviews. Oxford: Blackwell Publishing Ltd, 2008: 1-11.
[44] Schönherr J. Characterization of aqueous pores in plant cuticles and permeation of ionic solutes[J]. J Exp Bot,2006,57 (11):2471−2491. doi: 10.1093/jxb/erj217
[45] Eichert T,Kurtz A,Steiner U,Goldbach HE. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles[J]. Physiol Plant,2008,134 (1):151−160. doi: 10.1111/j.1399-3054.2008.01135.x
[46] Fernández V,Eichert T. Uptake of hydrophilic solutes through plant leaves:current state of knowledge and perspectives of foliar fertilization[J]. Crit Rev Plant Sci,2009,28 (1-2):36−68. doi: 10.1080/07352680902743069
[47] Ouyang XX,Ma J,Zhang R,Li P,Gao M,et al. Uptake of atmospherically deposited cadmium by leaves of vegetables:subcellular localization by NanoSIMS and potential risks[J]. J Hazard Mater,2022,431:128624. doi: 10.1016/j.jhazmat.2022.128624
[48] Chen DL,Yin S,Zhang XY,Lyu J,Zhang YR,et al. A high-resolution study of PM2.5 accumulation inside leaves in leaf stomata compared with non-stomatal areas using three-dimensional X-ray microscopy[J]. Sci Total Environ,2022,852:158543. doi: 10.1016/j.scitotenv.2022.158543
[49] Larue C,Castillo-Michel H,Sobanska S,Trcera N,Sorieul S,et al. Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure[J]. J Hazard Mater,2014,273:17−26. doi: 10.1016/j.jhazmat.2014.03.014
[50] 张罡,安海龙,史军娜,刘超,田菊,等. 欧美杨对不同粒径氧化锌颗粒物的吸附与吸收能力[J]. 北京林业大学学报,2017,39(4):46−54. doi: 10.13332/j.1000-1522.20160376 Zhang G,An HL,Shi JN,Liu C,Tian J,et al. Deposition and absorption capacity of Populus deltoides × P. nigra to different size zinc oxide aerosol[J]. Journal of Beijing Forestry University,2017,39 (4):46−54. doi: 10.13332/j.1000-1522.20160376
[51] 刘宇,张银龙. 植物叶面吸附与吸收PM2.5等颗粒物中重金属研究进展[J]. 世界林业研究,2022,35(4):8−13. Liu Y,Zhang YL. Research progress in heavy metals in PM2.5 and other atmospheric particulate matters retained and absorbed on plant leaf surface[J]. World Forestry Research,2022,35 (4):8−13.
[52] Burkhardt J,Basi S,Pariyar S,Hunsche M. Stomatal penetration by aqueous solutions-an update involving leaf surface particles[J]. New Phytol,2012,196 (3):774−787. doi: 10.1111/j.1469-8137.2012.04307.x
[53] Fernández V,Brown PH. From plant surface to plant metabolism:the uncertain fate of foliar-applied nutrients[J]. Front Plant Sci,2013,4:289.
[54] Luo J,Niu YD,Zhang Y,Zhang M,Tian YX,Zhou XL. Dynamic analysis of retention PM2.5 by plant leaves in rainfall weather conditions of six tree species[J]. Energy Sources Part A Recov Utilizat Environ Effects,2020,42 (8):1014−1025.
[55] Avellan A,Yun J,Morais BP,Clement ET,Rodrigues SM,Lowry GV. Critical review:role of inorganic nanoparticle properties on their foliar uptake and in Planta translocation[J]. Environ Sci Technol,2021,55 (20):13417−13431. doi: 10.1021/acs.est.1c00178
[56] Li LP,Zhang YQ,Ippolito JA,Xing WQ,Qiu KY,et al. Lead smelting effects heavy metal concentrations in soils,wheat,and potentially humans[J]. Environ Pollut,2020,257:113641. doi: 10.1016/j.envpol.2019.113641
[57] Yan BF,Nguyen C,Pokrovsky OS,Candaudap F,Coriou C,et al. Cadmium allocation to grains in durum wheat exposed to low Cd concentrations in hydroponics[J]. Ecotoxicol Environ Saf,2019,184:109592. doi: 10.1016/j.ecoenv.2019.109592
[58] Hu Y,Tian SK,Foyer CH,Hou DD,Wang HX,et al. Efficient phloem transport significantly remobilizes cadmium from old to young organs in a hyperaccumulator Sedum alfredii[J]. J Hazard Mater,2019,365:421−429. doi: 10.1016/j.jhazmat.2018.11.034
[59] Zhou WB,Qiu BS. Effects of cadmium hyperaccumulation on physiological characteristics of Sedum alfredii Hance (Crassulaceae)[J]. Plant Sci,2005,169 (4):737−745. doi: 10.1016/j.plantsci.2005.05.030
[60] Ma C,Liu FY,Xie P,Zhang K,Yang JX,et al. Mechanism of Pb absorption in wheat grains[J]. J Hazard Mater,2021,415:125618. doi: 10.1016/j.jhazmat.2021.125618
[61] Ma C,Xie P,Yang J,Lin L,Zhang K,Zhang HZ. Evaluating the contributions of leaf organ to wheat grain cadmium at the filling stage[J]. Sci Total Environ,2022,833:155217. doi: 10.1016/j.scitotenv.2022.155217
[62] Xu ZQ,Zhu Z,Zhao YH,Huang Z,Fei JC,et al. Foliar uptake,accumulation,and distribution of cadmium in rice (Oryza sativa L. ) at different stages in wet deposition conditions[J]. Environ Pollut,2022,306:119390. doi: 10.1016/j.envpol.2022.119390
[63] Večeřová K,Večeřa Z,Dočekal B,Oravec M,Pompeiano A,et al. Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles[J]. Environ Pollut,2016,218:207−218. doi: 10.1016/j.envpol.2016.05.013
[64] Dollard GJ. Glasshouse experiments on the uptake of foliar applied lead[J]. Environ Pollut Ser A Ecol Biol,1986,40 (2):109−119. doi: 10.1016/0143-1471(86)90078-4
[65] Xiong TT,Austruy A,Pierart A,Shahid M,Schreck E,et al. Kinetic study of phytotoxicity induced by foliar lead uptake for vegetables exposed to fine particles and implications for sustainable urban agriculture[J]. J Environ Sci,2016,46:16−27. doi: 10.1016/j.jes.2015.08.029
[66] Deng THB,Tang YT,van der Ent A,Sterckeman T,Echevarria G,et al. Nickel translocation via the phloem in the hyperaccumulator Noccaea caerulescens (Brassicaceae)[J]. Plant Soil,2016,404 (1-2):35−45. doi: 10.1007/s11104-016-2825-1
[67] Cakmak I,Welch RM,Hart J,Norvell WA,Oztürk L,et al. Uptake and retranslocation of leaf‐applied cadmium (109Cd) in diploid,tetraploid and hexaploid wheats[J]. J Exp Bot,2000,51 (343):221−226. doi: 10.1093/jexbot/51.343.221
[68] Wang JB,Su LY,Yang JZ,Yuan JG,Yin AG,et al. Comparisons of cadmium subcellular distribution and chemical forms between low-Cd and high-Cd accumulation genotypes of watercress (Nasturtium officinale L. R. Br. )[J]. Plant Soil,2015,396 (1):325−337.
[69] Schreck E,Foucault Y,Sarret G,Sobanska S,Cécillon L,et al. Metal and metalloid foliar uptake by various plant species exposed to atmospheric industrial fallout:mechanisms involved for lead[J]. Sci Total Environ,2012,427-428:253−262. doi: 10.1016/j.scitotenv.2012.03.051
[70] Schönherr J,Fernández V,Schreiber L. Rates of cuticular penetration of chelated FeⅢ:role of humidity,concentration,adjuvants,temperature,and type of chelate[J]. J Agric Food Chem,2005,53 (11):4484−4492. doi: 10.1021/jf050453t
[71] Fu XP,Dou CM,Chen YX,Chen XC,Shi JY,et al. Subcellular distribution and chemical forms of cadmium in Phytolacca americana L.[J]. J Hazard Mater,2011,186 (1):103−107. doi: 10.1016/j.jhazmat.2010.10.122
[72] Lindow SE,Brandl MT. Microbiology of the phyllosphere[J]. Appl Environ Microbiol,2003,69 (4):1875−1883. doi: 10.1128/AEM.69.4.1875-1883.2003
[73] Uzu G,Sobanska S,Sarret G,Muñoz M,Dumat C. Foliar lead uptake by lettuce exposed to atmospheric fallouts[J]. Environ Sci Technol,2010,44 (3):1036−1042. doi: 10.1021/es902190u
[74] Xie Y,Bu HS,Feng QJ,Wassie M,Amee M,et al. Identification of Cd-resistant microorganisms from heavy metal-contaminated soil and its potential in promoting the growth and Cd accumulation of bermudagrass[J]. Environ Res,2021,200:111730. doi: 10.1016/j.envres.2021.111730
[75] Villiers F,Ducruix C,Hugouvieux V,Jarno N,Ezan E,et al. Investigating the plant response to cadmium exposure by proteomic and metabolomic approaches[J]. Proteomics,2011,11 (9):1650−1663. doi: 10.1002/pmic.201000645
[76] Ackova DG. Heavy metals and their general toxicity for plants[J]. Plant Sci Today,2018,5 (1):14−18. doi: 10.14719/pst.2018.5.1.355
[77] Wang P,Lombi E,Zhao FJ,Kopittke PM. Nanotechnology:a new opportunity in plant sciences[J]. Trends Plant Sci,2016,21 (8):699−712. doi: 10.1016/j.tplants.2016.04.005
[78] Hong J,Wang C,Wagner DC,Gardea-Torresdey JL,He F,Rico CM. Foliar application of nanoparticles:mechanisms of absorption,transfer,and multiple impacts[J]. Environ Sci Nano,2021,8 (5):1196−1210. doi: 10.1039/D0EN01129K
[79] Wu J,Wang GY,Vijver MG,Bosker T,Peijnenburg WJGM. Foliar versus root exposure of AgNPs to lettuce:Phytotoxicity,antioxidant responses and internal translocation[J]. Environ Pollut,2020,261:114117. doi: 10.1016/j.envpol.2020.114117
[80] 刘程,蒋大程,宋晓旭,邱念伟,周峰. 叶绿素家族成员的结构差异与生物合成[J]. 植物生理学报,2020,56(3):356−366. doi: 10.13592/j.cnki.ppj.2019.0359 Liu C,Jiang DC,Song XX,Qiu NW,Zhou F. The structural differences and biosynthesis of chlorophyll family members[J]. Plant Physiology Journal,2020,56 (3):356−366. doi: 10.13592/j.cnki.ppj.2019.0359
[81] Saleh SR,Kandeel MM,Ghareeb D,Ghoneim TM,Talha NI,et al. Wheat biological responses to stress caused by cadmium,nickel and lead[J]. Sci Total Environ,2020,706:136013. doi: 10.1016/j.scitotenv.2019.136013
[82] Lu K,Shen DL,Liu XK,Dong SP,Jing XP,et al. Uptake of iron oxide nanoparticles inhibits the photosynthesis of the wheat after foliar exposure[J]. Chemosphere,2020,259:127445. doi: 10.1016/j.chemosphere.2020.127445
[83] Xiong TT,Zhang T,Dumat C,Sobanska S,Dappe V,et al. Airborne foliar transfer of particular metals in Lactuca sativa L.:translocation,phytotoxicity,and bioaccessibility[J]. Environ Sci Pollut Res,2019,26 (20):20064−20078. doi: 10.1007/s11356-018-3084-x
[84] Perreault F,Samadani M,Dewez D. Effect of soluble copper released from copper oxide nanoparticles solubilisation on growth and photosynthetic processes of Lemna gibba L.[J]. Nanotoxicology,2014,8 (4):374−382. doi: 10.3109/17435390.2013.789936
[85] Xie XL,He ZQ,Chen NF,Tang ZZ,Wang Q,Cai Y. The roles of environmental factors in regulation of oxidative stress in plant[J]. BioMed Res Int,2019,2019:9732325.
[86] 张梦如,杨玉梅,成蕴秀,周滔,段晓艳,等. 植物活性氧的产生及其作用和危害[J]. 西北植物学报,2014,34(9):1916−1926. Zhang MR,Yang YM,Cheng YX,Zhou T,Duan XY,et al. Generation of reactive oxygen species and their functions and deleterious effects in plants[J]. Acta Botanica Boreali-Occidentalia Sinica,2014,34 (9):1916−1926.
[87] Muzafffar A, Muzaffar U. Chapter8-Effects of dyshomeostasis of metals/metalloids on the generation of reactive oxygen and nitrogen species in plant tissues[M]//Aftab T, Hakeem K, eds. Metals Metalloids Soil Plant Water Systems. Cambridge: Academic Press, 2022: 185-198.
[88] Demidchik V. Mechanisms of oxidative stress in plants:from classical chemistry to cell biology[J]. Environ Exp Bot,2015,109:212−228. doi: 10.1016/j.envexpbot.2014.06.021
[89] Dubey S,Shri M,Gupta A,Rani V,Chakrabarty D. Toxicity and detoxification of heavy metals during plant growth and metabolism[J]. Environ Chem Lett,2018,16 (4):1169−1192. doi: 10.1007/s10311-018-0741-8
[90] Liu JG,Qu P,Zhang W,Dong Y,Li L,Wang MX. Variations among rice cultivars in subcellular distribution of Cd:the relationship between translocation and grain accumulation[J]. Environ Exp Bot,2014,107:25−31. doi: 10.1016/j.envexpbot.2014.05.004
[91] Weng BS,Xie XY,Weiss DJ,Liu JC,Lu HL,Yan CL. Kandelia obovata (S.,L. ) Yong tolerance mechanisms to Cadmium:subcellular distribution,chemical forms and thiol pools[J]. Mar Pollut Bull,2012,64 (11):2453−2460. doi: 10.1016/j.marpolbul.2012.07.047
[92] Hasanuzzaman M,Bhuyan MHMB,Zulfiqar F,Raza A,Mohsin SM,et al. Reactive oxygen species and antioxidant defense in plants under abiotic stress:revisiting the crucial role of a universal defense regulator[J]. Antioxidants,2020,9 (8):681. doi: 10.3390/antiox9080681
[93] Pandita D. Chapter17-Reactive oxygen and nitrogen species: Antioxidant defense studies in plants[M]//Aftab T, Roychoudhury A, eds. Plant Perspectives to Global Climate Changes. Cambridge: Academic Press, 2022: 355-371.
[94] Sharma P,Jha AB,Dubey RS,Pessarakli M. Reactive oxygen species,oxidative damage,and antioxidative defense mechanism in plants under stressful conditions[J]. J Bot,2012,2012:217037.
[95] Hasanuzzaman M,Bhuyan MHMB,Anee TI,Parvin K,Nahar K,et al. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress[J]. Antioxidants (Basel)
,2019,8 (9):384. doi: 10.3390/antiox8090384 -
期刊类型引用(6)
1. 庞婷婷,金紫依,高家晖,潘钰,肖彩玲,陈路锋,曹梦西,刘艳伟,阴永光,梁勇. 基于电感耦合等离子体质谱(ICP-MS)法的大棚和露天种植模式下典型叶类蔬菜重金属累积特征. 中国无机分析化学. 2025(03): 330-339 .  百度学术
百度学术
2. 曾婉珍,艾灵,吴秋霞,张玉,朱晶晶,倪祥银,吴福忠. 亚热带森林72个常绿和落叶树种凋落叶重金属的富集效率. 中国科学:生命科学. 2025(03): 563-574 .  百度学术
百度学术
3. 林跃胜,王飞,郭尔洛,李鹤冉,方凤满. 菜子湖湿地土壤-植物体系重金属富集、转运及影响因素分析. 环境科学学报. 2025(03): 373-383 .  百度学术
百度学术
4. 曹敬文,徐照,刘月月,周袁媛,季涛涛,李桃生,程雄卫. 利用ICP-MS和LA-ICP-TOF-MS探究上海青中铅的富集与转运特征. 化学试剂. 2024(04): 80-87 .  百度学术
百度学术
5. 杨乾巍,杨迪,张良,杜光映,张明星,何愿子,唐桐桐,赵雅秋. 我国47种中药材中重金属含量分析与数据挖掘. 中国现代中药. 2024(04): 625-634 .  百度学术
百度学术
6. 包成龙,刘根红,吴炫燕,李杨,郑国琦,陈璐. 重金属污染对枸杞生长及品质影响的研究进展. 北方园艺. 2024(16): 125-132 .  百度学术
百度学术
其他类型引用(3)



 下载:
下载: