Assessing microbial diversity in anthers of Eriobotrya japonica (Thunb.) Lindl. based on high-throughput sequencing
-
摘要:
果树具有较高的经济价值,并大多依赖传粉者实现繁殖成功以保证产量。花药微生物能影响植物的花粉活力,其组成也可能受到传粉者访问的影响;理解花药微生物的多样性和群落构建模式对于提高果树的繁殖适合度具有潜在意义,但目前相关研究仍相对缺乏。本研究基于高通量测序技术分析了传粉者访问前(套袋组)与访问后(访问组)枇杷(Eriobotrya japonica (Thunb.) Lindl.)花药中真菌与细菌的组成及差异。结果显示,传粉者访问未显著改变花药微生物的多样性及组成,套袋组中优势真菌科为枝孢菌科与球腔菌科,访问组优势真菌科则为枝孢菌科与梅奇酵母科。此外,两组中优势细菌科均为产碱杆菌科与欧文菌科。研究结果表明,枇杷花药微生物具有相对稳定的群落结构,传粉者不是驱动花药微生物群落构建的主要因素。
Abstract:Fruit trees, vital to global agriculture, depend heavily on pollinators to facilitate successful reproduction and ensure optimal yield. Microorganisms associated with anthers can influence pollen viability, and their community composition may be affected by pollinator activity. While understanding the diversity and community assembly patterns of these microorganisms has potential implications for enhancing the reproductive fitness of fruit trees, research in this area remains relatively scarce. This study employed high-throughput sequencing to analyze the diversity and community structure of fungi and bacteria on the anthers of loquat (Eriobotrya japonica (Thunb.) Lindl.) before (bagged group) and after (nature group) pollinator visitation. Results showed that pollinator visitation had no significant effect on the diversity or composition of anther microbiomes. In the bagged group, dominant fungal taxa included Cladosporiaceae and Mycosphaerellaceae, whereas Cladosporiaceae and Metschnikowiaceae were dominant in the nature group. Bacterial communities in both groups were also dominated by Alcaligenaceae and Erwiniaceae. These findings indicate that the microbial community composition within loquat anthers is inherently stable and largely unaffected by pollinator activity.
-
花,作为植物的繁殖器官,主要由花瓣、雄蕊、雌蕊、花托等组织构成。不同种类植物的花在结构上存在差异,这些组织和结构的独特性和完整性对于植物实现传粉与繁殖成功至关重要。研究发现,大约90%的开花植物依赖动物传粉,随着全球传粉者多样性的下降[1-5],如何保证植物能够获得充足的传粉服务已成为一个重要的问题[6]。近年来,关于植物、花微生物与传粉者之间相互作用的研究日益受到关注,研究认为花微生物在维持植物与传粉者相互作用关系中起到了重要作用[7]。花为微生物提供丰富的营养物质和适宜生长的环境,有利于微生物的定殖[8]。定殖于花上的微生物通过干扰花粉萌发、花粉管生长以及果实发育等过程,直接影响植物的有性生殖过程[9-11];也可以通过改变花信号,影响传粉者行为及组成,间接影响植物传粉成功[12-15]。为研究花微生物对植物传粉与繁殖成效的影响,首先需要了解花微生物的组成及群落构建过程。花表面承载着丰富的微生物群落,包括细菌、真菌和病毒等[16-21]。根据微生物的来源,可以将其分为非生物来源与生物来源。非生物来源的微生物主要指来自空气、土壤及雨水等自然环境因素的微生物[22],而生物来源的微生物则主要包括传粉者携带并移入花中的微生物以及植物自身内源性的微生物。因此,花表面微生物的群落组成受到植物自身特性、环境因素及传粉者访问等因素的共同影响[23, 24]。研究植物与传粉者等因素对花微生物群落构建的影响,有助于深入理解植物、花微生物与传粉者之间的三方互作关系。
在花微生物的相关研究中,研究者发现花蜜微生物可以影响植物的传粉与繁殖成功,花蜜微生物多样性及功能的研究已成为该领域热点之一[9-15],而对花药微生物多样性及功能的研究相对较少。花药在植物繁殖过程中发挥着重要作用,其表面也通常较为粗糙,便于微生物的定殖[25]。花药同时也是一些植物病原体易感染的部位,容易引起植物居群内产生严重的与花粉相关的病害[26, 27],例如微球黑粉菌(Microbotryum)可以在石竹科植物的花药上快速繁殖,而传粉者作为花粉与病原菌的载体,可以将病原菌扩散到新的植株上,最终导致整个居群的感染[28-31]。Curran等[32]的研究表明,花药感染黑粉菌后,酢浆草属(Oxalis)植物的结籽数显著下降。同时,一些与花粉或柱头相关的微生物可能进入宿主的繁殖过程,作为种子内生菌传播并感染下一代[33]。因此,理解花药微生物的组成及群落构建模式对于提高植物的繁殖适合度具有潜在意义。作为冬季开花的果树,枇杷(Eriobotrya japonica (Thunb.) Lindl.)为冬季传粉者提供了重要的蜜粉资源,同时枇杷依赖传粉者传递花粉以实现繁殖成功[34]。枇杷单花约含20枚花药,可以释放大量花粉。研究表明,枇杷在花期和果期容易感染多种真菌与细菌病害[35, 36],造成枇杷果实产量与品质的下降,然而目前相关研究仍相对缺乏。
在以往的研究中,研究者主要使用传统的平板培养技术统计花微生物的发生率和密度[16, 37, 38],但这种方法存在一定的限局性,如无法培养出对环境或营养条件有特殊需求的微生物。随着科技的发展,高通量测序技术在环境科学、食品工业等领域得到广泛应用,可以帮助研究者更准确地解析样本中微生物的种类及相对含量,并全面分析微生物群落的多样性以及分布规律[39, 40]。本文拟通过高通量测序技术探究枇杷花药中真菌与细菌的群落组成模式,以期为该物种繁殖过程中多物种的互作研究提供参考资料。
1. 材料与方法
1.1 实验材料
枇杷属于蔷薇科枇杷属(Eriobotrya),为常绿小乔木,适宜在温暖环境中生长,在四川、贵州、湖北等地被广泛种植。枇杷单个花序上可以生长约100~200朵花,每朵花都具有蜜腺、5个花柱以及约20个花药。本文研究地点位于湖北省武汉市武汉大学校内(30°32′06″N,114°21′19″E,海拔82.5 m),该区域人工种植枇杷植株的花期从11月持续至次年1月,果期大约在5月左右。枇杷的单花期最长可达7 d,柱头在开花第1天内即具有可授性,而花药在开花第2天逐渐纵裂并释放花粉。枇杷花的访问者包括中华蜜蜂(Apis cerana),墨胸胡蜂(Vespa velutina)以及红头丽蝇(Calliphora vicina)等。在较为温暖的11月份,传粉昆虫的访花高峰期为9:00-16:00,并且受到温度的影响,每朵枇杷单花在10 min内接受传粉昆虫的访问次数范围为0.1~0.4次[34]。
1.2 实验方法
1.2.1 花药的收集
为研究传粉者对枇杷花药微生物群落组成的影响,设置套袋组与访问组。套袋组使用尼龙网纱袋对花序进行套袋处理,以此排除传粉者的访问;访问组则不进行套袋处理,允许传粉者自由访问枇杷单花。在正式实验开始前,移除实验组内已开放的枇杷花,以保证两组中花期的一致性。于2021年11月,在6个枇杷植株上随机选取60个花序分别作为对照组(30个花序)与访问组(30个花序),每个花序中至少含有2~3朵枇杷单花。优先选取单花期在第3天的枇杷花作为实验样本(花药已全部开裂),此阶段访问组的枇杷花在天气晴朗时更可能接收到传粉者访问。使用消毒后的剪刀收集套袋组与访问组枇杷单花,分别放入网纱袋中,并就地取样。随后使用消毒后的镊子挑取花药放入EP管中,将20朵枇杷单花的全部花药作为一个样本,套袋组与访问组各收集4个样本。为了消除天气、温度等环境因素对花药微生物的组成产生的潜在影响,在一周内对花药进行多次取样,减少随机误差,提高数据的准确性。将收集的花药样本放置于冰盒中,随即带回实验室置于−80 ℃的冰箱中冷冻保存,防止微生物的继续增殖;最后,将花药样本寄往美吉公司进行扩增子测序(16S与ITS测序)。
1.2.2 数据分析
为比较传粉者访问对花药微生物Alpha多样性的影响,使用Wilcoxon秩和检验分别比较套袋组与访问组中细菌与真菌的Alpha多样性,并使用BH进行多重检验校正。此外,为了评估传粉者访问对花药微生物组成的影响,分别计算组内及组间的Bray-Curtis、Unweighted-UniFrac和Weighted-UniFrac距离,并使用置换多元方差分析(PERMANOVA)进行差异性检验。最后,通过非度量多维尺度(NMDS)对套袋组与访问组细菌和真菌组成的差异进行可视化呈现。
2. 结果与分析
2.1 花药微生物稀释曲线
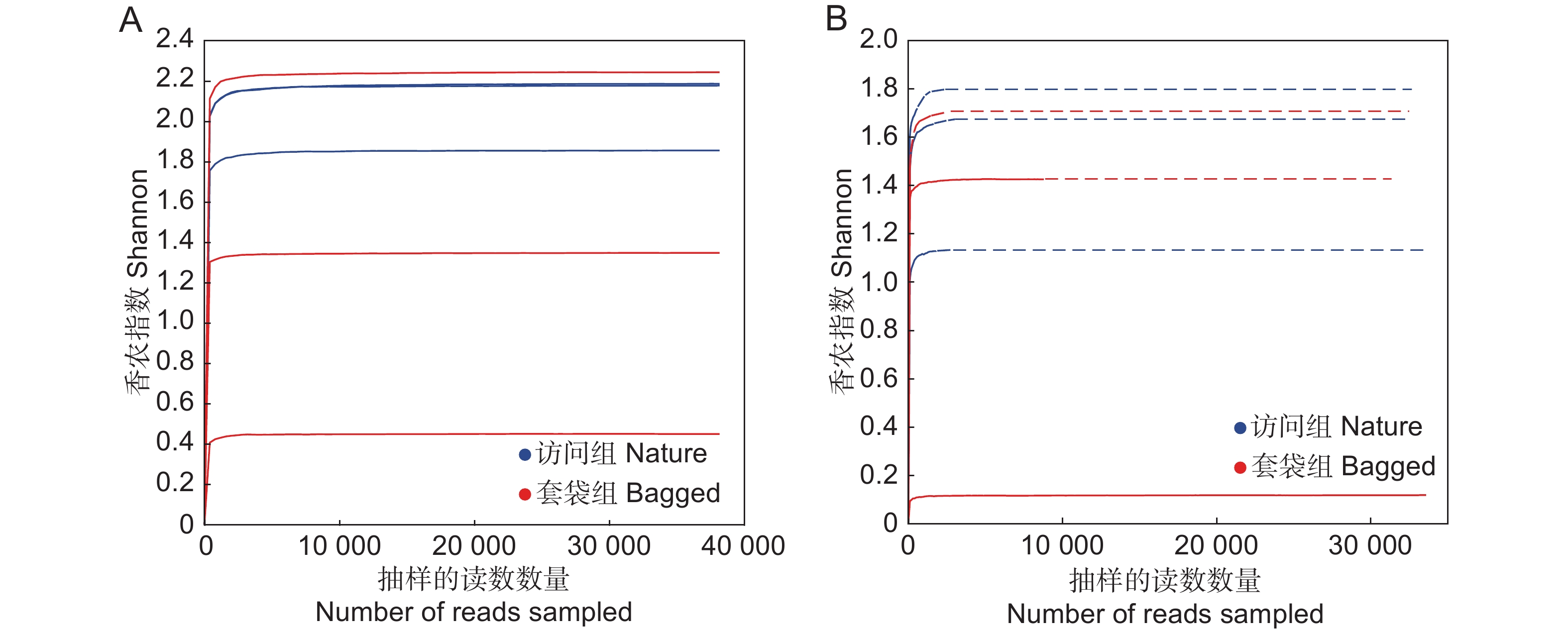
基于Shannon指数绘制套袋组与访问组中花药真菌与细菌的稀释曲线。结果显示,随着测序深度的加深,两组处理中花药真菌与细菌稀释曲线趋于平缓,并最终达到饱和状态(图1)。表明在该测序深度下,绝大多数的真菌与细菌均已被检测到。因此,测序结果可以反映枇杷花药在接受传粉者访问前后的微生物的组成及多样性。
![]() 图 1 枇杷花药微生物群落稀释曲线基于香农指数(Shannon)展示枇杷套袋组(红色)与访问组(蓝色)花药真菌(A)与细菌(B)群落的稀释曲线。Figure 1. Rarefaction curves of microbial community in anthers of Eriobotrya japonica based on Shannon indexRarefaction curves based on Shannon index, showing fungal (A) and bacterial (B) diversity in bagged (red) and nature (blue) groups.
图 1 枇杷花药微生物群落稀释曲线基于香农指数(Shannon)展示枇杷套袋组(红色)与访问组(蓝色)花药真菌(A)与细菌(B)群落的稀释曲线。Figure 1. Rarefaction curves of microbial community in anthers of Eriobotrya japonica based on Shannon indexRarefaction curves based on Shannon index, showing fungal (A) and bacterial (B) diversity in bagged (red) and nature (blue) groups.2.2 花药微生物群落组成
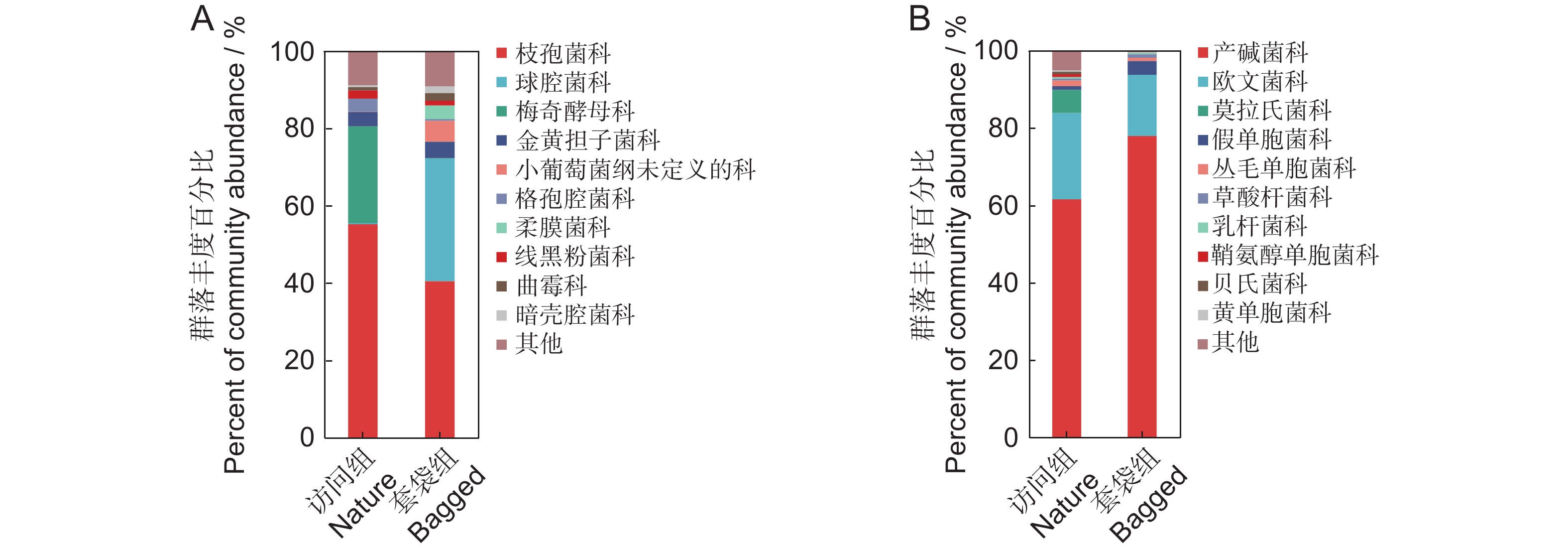
根据丰度对花药微生物进行排序,并在科的水平上列举排名前10的微生物物种,其余物种合并为“Others”(图2)。套袋组中优势细菌为产碱杆菌科、欧文菌科以及假单胞菌科,平均相对丰度分别为77.96%、15.80%和3.59%,合计占细菌总丰度的97.35%;访问组中优势细菌为产碱杆菌科、欧文菌科以及莫拉氏菌科,平均相对丰度分别为61.56%、22.36%和5.96%,合计占细菌总丰度的89.88%。套袋组中优势真菌为枝孢菌科、球腔菌科以及微球黑粉菌目下未定义的科,平均相对丰度分别为40.49%、31.74%和5.40%,占真菌总丰度的77.63%;访问组中优势真菌为枝孢菌科、梅奇酵母科以及短梗霉科,平均相对丰度分别为55.20%、25.01%和3.74%,占真菌总丰度的83.7%。对于花药细菌而言,两个实验组中都以产碱杆菌科与欧文菌科作为主要的两种细菌科,而在真菌方面,两个实验组中枝孢菌科都具有较高的占比。两个实验组中最优势的微生物均为同一科的微生物,因此花药中具有相对稳定的微生物群落。
![]() 图 2 枇杷花药微生物群落结构(科水平)A:以ITS2测序表征的平均丰度>1%的套袋组与访问组真菌科组成;B:以16S测序表征的平均丰度>1%的套袋组与访问组细菌科组成。图中仅展示各实验组中丰度占比前10的微生物,其余物种合并为“其他”。Figure 2. Structure of microbial community in anthers of Eriobotrya japonica (family level)A: Composition of fungal families with an average abundance greater than 1%, characterized by ITS2 sequencing, in nature and bagged groups; B: Composition of bacterial families with an average abundance greater than 1%, characterized by 16S sequencing, in nature and bagged groups. Only the top 10 most abundant microbes in each experimental group are shown in the figure, with all other species combined under “Others”.
图 2 枇杷花药微生物群落结构(科水平)A:以ITS2测序表征的平均丰度>1%的套袋组与访问组真菌科组成;B:以16S测序表征的平均丰度>1%的套袋组与访问组细菌科组成。图中仅展示各实验组中丰度占比前10的微生物,其余物种合并为“其他”。Figure 2. Structure of microbial community in anthers of Eriobotrya japonica (family level)A: Composition of fungal families with an average abundance greater than 1%, characterized by ITS2 sequencing, in nature and bagged groups; B: Composition of bacterial families with an average abundance greater than 1%, characterized by 16S sequencing, in nature and bagged groups. Only the top 10 most abundant microbes in each experimental group are shown in the figure, with all other species combined under “Others”.2.3 花药微生物多样性差异
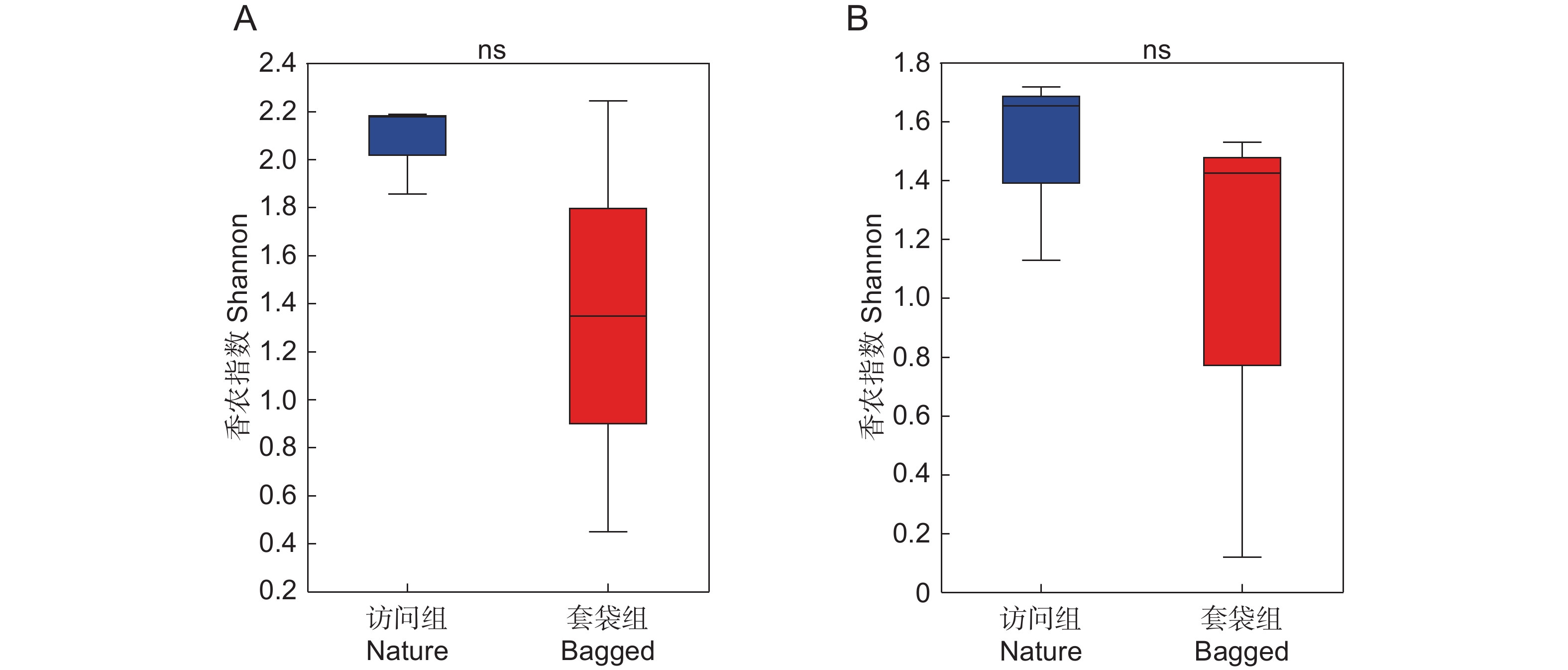
基于Shannon指数计算套袋组与访问组花药中细菌与真菌群落的Alpha多样性,并比较两组之间的差异。结果显示,与套袋组相比,访问组中细菌与真菌的Alpha多样性均呈增加趋势,但在统计学上并未达到显著性水平(Wilcoxon秩和检验,BH校正,P细菌=0.993 8,P真菌=0.662 5,图3)。此外,本文计算了Chao以及Coverage指数,从微生物群落的丰富度和覆盖度角度分析套袋处理对细菌和真菌群落Alpha多样性的影响。基于Chao与Coverage指数对比两组Alpha多样性差异,发现套袋处理对微生物群落Alpha多样性没有显著影响,因此并未在本文中呈现详细数据。
![]() 图 3 枇杷花药微生物Alpha多样性分析(Shannon指数)基于香农指数展示枇杷访问组(蓝色)与套袋组(红色)花药真菌(A)与细菌(B)的Alpha多样性。利用Wilcoxon秩和检验比较两个实验组中真菌与细菌Alpha多样性的差异,箱线图显示了中值和四分位距,ns表示两个实验组间不存在显著差异。Figure 3. Alpha diversity analysis (Shannon index) of microbial community in anthers of Eriobotrya japonicaAlpha diversity of fungal (A) and bacterial (B) communities in anthers of Eriobotrya japonica in nature (blue) and bagged (red) groups is presented based on Shannon index. Differences in fungal and bacterial alpha diversity between two experimental groups were assessed using Wilcoxon rank-sum test. Boxplot illustrates median and interquartile range, with “ns” signifying no significant difference.
图 3 枇杷花药微生物Alpha多样性分析(Shannon指数)基于香农指数展示枇杷访问组(蓝色)与套袋组(红色)花药真菌(A)与细菌(B)的Alpha多样性。利用Wilcoxon秩和检验比较两个实验组中真菌与细菌Alpha多样性的差异,箱线图显示了中值和四分位距,ns表示两个实验组间不存在显著差异。Figure 3. Alpha diversity analysis (Shannon index) of microbial community in anthers of Eriobotrya japonicaAlpha diversity of fungal (A) and bacterial (B) communities in anthers of Eriobotrya japonica in nature (blue) and bagged (red) groups is presented based on Shannon index. Differences in fungal and bacterial alpha diversity between two experimental groups were assessed using Wilcoxon rank-sum test. Boxplot illustrates median and interquartile range, with “ns” signifying no significant difference.2.4 花药微生物组成差异
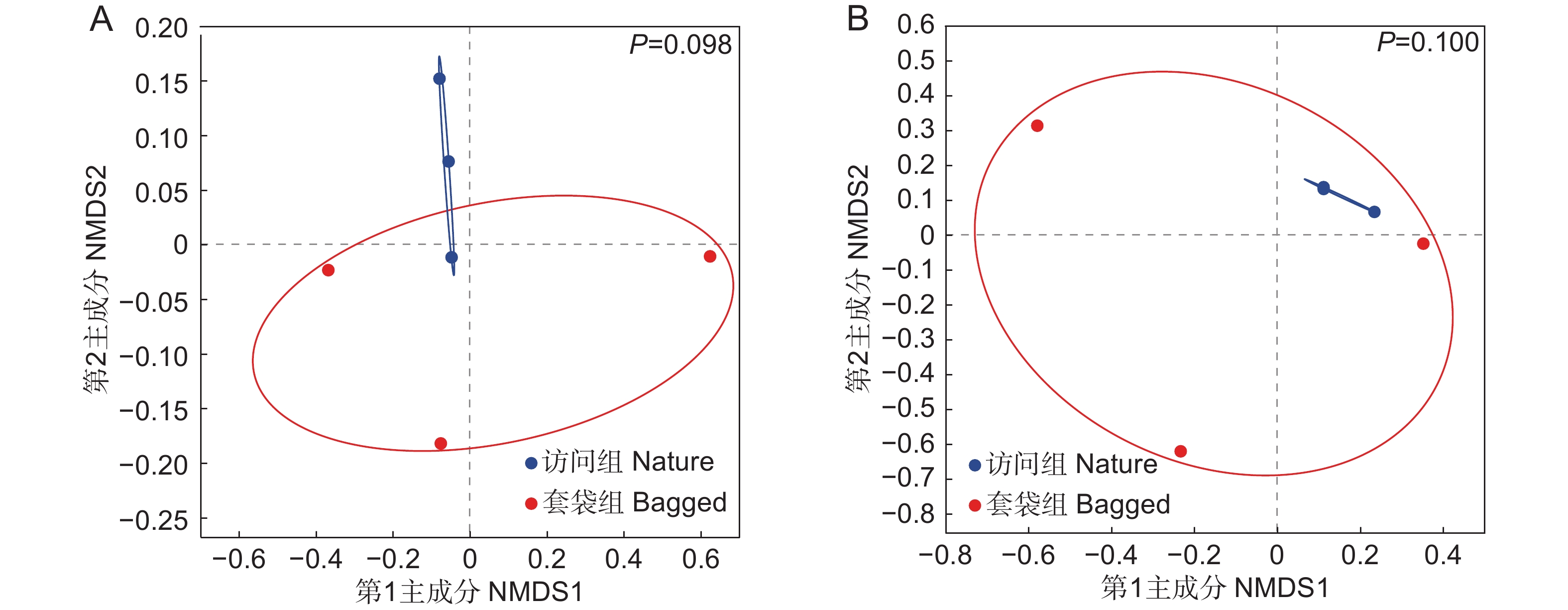
基于Bray-Curtis距离,对套袋组和访问组枇杷花药微生物群落进行了NMDS排序。结果表明,两个实验组中细菌与真菌群落的组成没有显著差异(图4),即套袋处理不会改变花药微生物的群落组成。Bray-Curtis距离算法仅考虑每个样本中微生物的相对丰度,Unweighted-UniFrac距离算法则只关注微生物分类单元的进化关系,Weighted-UniFrac距离算法综合考虑了微生物的相对丰度及分类单元的进化关系。在使用不同的距离算法进一步分析套袋处理对微生物分类单元进化关系的影响时,结果与Bray-Curtis距离算法相一致(表1)。因此,花药微生物群落组成具有较高的稳定性,不受套袋处理的影响。
![]() 图 4 枇杷花药微生物群落的NMDS分析基于Bray-Curtis距离对枇杷花药微生物群落进行非度量多维尺度(NMDS)排序,对真菌(A)和细菌(B)进行了单独排序。蓝色实心圆圈代表访问组样本,红色实心圆圈代表套袋组样本。绘制分组椭圆展示样本点之间的分布情况。基于Bray-Curtis距离算法,利用PERMANOVA比较套袋组与访问组真菌与细菌群落组成,P<0.05表示具有显著差异。Figure 4. Non-metric multidimensional scaling (NMDS) of microbial community in anthers of Eriobotrya japonicaNon-metric multidimensional scaling (NMDS) ordination of microbial communities in anthers of Eriobotrya japonica based on Bray-Curtis distance was performed, with separate analyses for fungi (A) and bacteria (B). Blue solid circles represent samples from nature group, red solid circles represent samples from bagged group. Group ellipses are drawn to present distribution of sample points. Based on Bray-Curtis distance algorithm, PERMANOVA was used to compare fungal and bacterial community compositions between bagged and nature groups, with P<0.05 indicating significant differences.表 1 使用不同距离算法的PERMANOVA分析汇总Table 1. Summary of permutation multivariate analysis of variance (PERMANOVA) using different distance algorithms
图 4 枇杷花药微生物群落的NMDS分析基于Bray-Curtis距离对枇杷花药微生物群落进行非度量多维尺度(NMDS)排序,对真菌(A)和细菌(B)进行了单独排序。蓝色实心圆圈代表访问组样本,红色实心圆圈代表套袋组样本。绘制分组椭圆展示样本点之间的分布情况。基于Bray-Curtis距离算法,利用PERMANOVA比较套袋组与访问组真菌与细菌群落组成,P<0.05表示具有显著差异。Figure 4. Non-metric multidimensional scaling (NMDS) of microbial community in anthers of Eriobotrya japonicaNon-metric multidimensional scaling (NMDS) ordination of microbial communities in anthers of Eriobotrya japonica based on Bray-Curtis distance was performed, with separate analyses for fungi (A) and bacteria (B). Blue solid circles represent samples from nature group, red solid circles represent samples from bagged group. Group ellipses are drawn to present distribution of sample points. Based on Bray-Curtis distance algorithm, PERMANOVA was used to compare fungal and bacterial community compositions between bagged and nature groups, with P<0.05 indicating significant differences.表 1 使用不同距离算法的PERMANOVA分析汇总Table 1. Summary of permutation multivariate analysis of variance (PERMANOVA) using different distance algorithmsGroup df Bray-Curtis Unweighted-UniFrac Weighted-UniFrac F R2 P F R2 P F R2 P 真菌 1 1.423 0.262 0.098 1.387 0.257 0.100 1.233 0.236 0.200 细菌 1 2.167 0.351 0.100 0.991 0.198 0.500 0.473 0.106 0.900 3. 讨论
花粉的“健康”直接影响植物的繁殖成功,因此维护花药内部环境的稳定性至关重要。研究表明,当花药感染某些微生物后,会对植物的生殖适合度产生消极影响[32],同时传粉者访问会改变花微生物的群落组成[41]。本文通过高通量测序技术探究了传粉者访问前(套袋组)与传粉者访问后(访问组),人工种植的枇杷花药微生物的组成及其变化。结果表明,两组花药微生物的Alpha多样性不存在显著差异,并且最优势的花药微生物属于同一个科。在细菌方面,两组均以产碱杆菌科和欧文菌科为主要细菌;而在真菌方面,枝孢菌科在两组中具有较高的占比。
本文利用不同距离算法比较了套袋组与访问组微生物群落组成,发现花药微生物群落组成具有较强的稳定性,并且传粉者不是驱动花药微生物群落构建的主要因素。研究普遍认为,传粉者的访问会改变花微生物群落的组成[21, 42, 43],Pozo等[41]在6个不同海拔研究了同一植物的酵母发生率与丰度,发现传粉者的组成与活动对花微生物群落的构建具有显著影响。然而,Rebolleda Gómez等[23]研究了花部位、传粉者及不同居群对多斑沟酸浆(Mimulus guttatus Fisch. ex DC.)花微生物群落组成的影响,发现不同花部位和不同居群之间的微生物群落组成具有显著差异,而传粉者的访问对微生物组成并没有显著影响。因此,本研究认为花的不同部位存在过滤机制,并且是影响微生物群落构建的主要因素。研究者已经发现叶片及花蜜对微生物的定殖存在过滤机制,而对花药微生物是否存在过滤机制的研究相对较少。Vokou等[44]比较了空气细菌与9种植物叶片附生细菌组成的相似性,发现只有少数空气细菌适应叶片环境并定殖。花蜜则通过高渗透压、高糖浓度等生理特性,限制了微生物的定殖[38, 45]。Junker等[46]利用高通量测序技术对比分析了多型铁心木(Metrosideros polymorpha Gaudich.)叶片、花药、花蜜以及柱头细菌群落的组成,发现与海拔,地理距离等因素相比,不同部位潜在的过滤机制是影响微生物群落组成的主要因素。Ambika Manirajan等[33]比较了4种植物花粉微生物的组成,结果显示不同花粉的微生物组成具有显著差异,并认为不同植物花粉独特的营养水平与结构特征,导致了这种差异。Kosenko等[47]利用扫描电镜解析了34种植物花粉的超微结构,发现不同植物(属或种)花粉粒外层的形状与结构具有显著差异,侧面印证了Ambika Manirajan等[33]的猜测。因此,花药存在过滤机制影响其表面微生物的群落构建。此外,有研究表明花蜜与花柱微生物存在优先效应,即优先定殖的微生物抢占营养及空间资源,进而限制后续微生物定殖[13, 48-50]。由于优先效应的存在,花蜜与花柱微生物物种多样性较低,微生物组成由单一的优势酵母与细菌为主导[45, 51],这与本文花药的研究结果一致。在本研究中,尽管传粉者的访问会改变某些微生物的相对含量(引入新的微生物),但整体的微生物群落组成并未发生显著变化,因此我们认为花药存在的过滤机制及花药微生物存在的优先效应可能是枇杷花药微生物群落构建的主要因素。
由于实验条件限制,本研究仅使用扩增子测序技术探索了传粉者访问前后,枇杷花药中微生物的多样性,而未通过传统的培养技术获得花药中优势微生物,并进一步探索微生物的功能。研究表明,花蜜微生物会通过直接或间接的方式影响植物的传粉与繁殖成效[9-15]。例如微生物通过改变视觉、嗅觉或味觉信号来影响传粉者的访问行为,进而影响植物的繁殖成效[15, 52-55],或是直接干扰柱头花粉萌发影响植物的繁殖过程[13]。与花蜜微生物功能类似,花药微生物可能存在潜在的功能,但对于花药上真菌和细菌群落如何影响传粉后植物的繁殖成功还未见报道。
此外,枇杷花具有多种传粉者,我们未探讨这些传粉者携带的微生物是否具有差异,携带的微生物是否会定殖在花药上并产生新的花信号,进而对枇杷的传粉与繁殖成效产生不同的影响。Morris等[56]比较了柳叶菜属植物(Epilobium canum (Greene) P. H. Raven.)在接受蜂鸟访问以及木蜂盗取花蜜后,花蜜微生物群落组成的差异,发现花蜜在接受蜂鸟与木蜂访问后,微生物丰度显著增加,并且在木蜂盗取花蜜后,花蜜微生物丰度是蜂鸟访问后花蜜微生物丰度的10倍。此外,与蜂鸟访问后的花蜜相比,木蜂实施盗取行为后的花蜜中,醋酸菌科与梅奇酵母科成为新的优势微生物。作者因此认为传粉者类型也是驱动花蜜微生物群落构建的主要因素。
花药微生物群落组成同样受到植物种类的影响[33, 46]。Ambika Manirajan等[33]利用高通量测序技术检测了垂枝桦(Betula pendula Roth.)、黑麦(Secale cereale L.)、欧洲油菜(Brassica napus L.)、秋水仙(Colchicum autumnale L.)花粉细菌的群落组成,发现垂枝桦、欧洲油菜、秋水仙的花粉微生物以肠杆菌科为主,而微球菌科和诺卡氏菌科是黑麦花粉中的优势微生物。Junker等[46]测定了多型铁心木花药微生物的组成,同样发现肠杆菌科是多型铁心木花药的优势细菌。因此,肠杆菌科微生物可能是花药中较为常见的一类细菌。在本文中,枇杷花药微生物中以产碱杆菌、欧文菌科为优势细菌,这与Ambika Manirajan[33]、Junker等[46]的研究结果具有差异。我们认为本文的研究对象枇杷,其传粉者与传粉类型(依靠中华蜜蜂、墨胸胡蜂等进行虫媒传粉)与上述5种植物的传粉者与传粉类型存在差异(风媒传粉或依靠西部黄胡蜂等传粉者进行虫媒传粉),进而导致优势微生物种类存在差异。此外,本文是在武汉大学校园内对枇杷花药微生物群落进行的相关研究,该区域枇杷定期接受人工管理,因此其微生物组成也可能受到人类行为的影响。目前也有研究探讨了果树花蜜微生物的群落组成及其对植物繁殖适合度的影响[39, 40, 57]。将本文花药微生物的群落组成与上述文献对比,发现在科的水平上,枇杷花药优势微生物与苹果(Malus domestica Borkh.)、西洋梨(Pyrus communis L.)、高丛越橘(Vaccinium corymbosum L.)、番茄(Solanum lycopersicum L.)的花蜜优势微生物不属于同一科,该结果可能是由于不同花部位的过滤机制导致;而梨(Pyrus)、蓝莓(Vaccinium uliginosum L.)、番茄中花蜜优势微生物也不相同,该结果可能是由于植物种类不同导致。对于花药微生物群落组成机制,以及微生物潜在功能的探讨,有助于深入理解植物、花微生物与传粉者之间的三方互作关系,并为果树繁殖过程中微生物的多样性及生态功能的研究提供参考资料。
-
图 1 枇杷花药微生物群落稀释曲线
基于香农指数(Shannon)展示枇杷套袋组(红色)与访问组(蓝色)花药真菌(A)与细菌(B)群落的稀释曲线。
Figure 1. Rarefaction curves of microbial community in anthers of Eriobotrya japonica based on Shannon index
Rarefaction curves based on Shannon index, showing fungal (A) and bacterial (B) diversity in bagged (red) and nature (blue) groups.
图 2 枇杷花药微生物群落结构(科水平)
A:以ITS2测序表征的平均丰度>1%的套袋组与访问组真菌科组成;B:以16S测序表征的平均丰度>1%的套袋组与访问组细菌科组成。图中仅展示各实验组中丰度占比前10的微生物,其余物种合并为“其他”。
Figure 2. Structure of microbial community in anthers of Eriobotrya japonica (family level)
A: Composition of fungal families with an average abundance greater than 1%, characterized by ITS2 sequencing, in nature and bagged groups; B: Composition of bacterial families with an average abundance greater than 1%, characterized by 16S sequencing, in nature and bagged groups. Only the top 10 most abundant microbes in each experimental group are shown in the figure, with all other species combined under “Others”.
图 3 枇杷花药微生物Alpha多样性分析(Shannon指数)
基于香农指数展示枇杷访问组(蓝色)与套袋组(红色)花药真菌(A)与细菌(B)的Alpha多样性。利用Wilcoxon秩和检验比较两个实验组中真菌与细菌Alpha多样性的差异,箱线图显示了中值和四分位距,ns表示两个实验组间不存在显著差异。
Figure 3. Alpha diversity analysis (Shannon index) of microbial community in anthers of Eriobotrya japonica
Alpha diversity of fungal (A) and bacterial (B) communities in anthers of Eriobotrya japonica in nature (blue) and bagged (red) groups is presented based on Shannon index. Differences in fungal and bacterial alpha diversity between two experimental groups were assessed using Wilcoxon rank-sum test. Boxplot illustrates median and interquartile range, with “ns” signifying no significant difference.
图 4 枇杷花药微生物群落的NMDS分析
基于Bray-Curtis距离对枇杷花药微生物群落进行非度量多维尺度(NMDS)排序,对真菌(A)和细菌(B)进行了单独排序。蓝色实心圆圈代表访问组样本,红色实心圆圈代表套袋组样本。绘制分组椭圆展示样本点之间的分布情况。基于Bray-Curtis距离算法,利用PERMANOVA比较套袋组与访问组真菌与细菌群落组成,P<0.05表示具有显著差异。
Figure 4. Non-metric multidimensional scaling (NMDS) of microbial community in anthers of Eriobotrya japonica
Non-metric multidimensional scaling (NMDS) ordination of microbial communities in anthers of Eriobotrya japonica based on Bray-Curtis distance was performed, with separate analyses for fungi (A) and bacteria (B). Blue solid circles represent samples from nature group, red solid circles represent samples from bagged group. Group ellipses are drawn to present distribution of sample points. Based on Bray-Curtis distance algorithm, PERMANOVA was used to compare fungal and bacterial community compositions between bagged and nature groups, with P<0.05 indicating significant differences.
表 1 使用不同距离算法的PERMANOVA分析汇总
Table 1 Summary of permutation multivariate analysis of variance (PERMANOVA) using different distance algorithms
Group df Bray-Curtis Unweighted-UniFrac Weighted-UniFrac F R2 P F R2 P F R2 P 真菌 1 1.423 0.262 0.098 1.387 0.257 0.100 1.233 0.236 0.200 细菌 1 2.167 0.351 0.100 0.991 0.198 0.500 0.473 0.106 0.900 -
[1] Theodorou P,Radzevičiūtė R,Lentendu G,Kahnt B,Husemann M,et al. Urban areas as hotspots for bees and pollination but not a panacea for all insects[J]. Nat Commun,2020,11(1):576. doi: 10.1038/s41467-020-14496-6
[2] Didham RK,Basset Y,Collins CM,Leather SR,Littlewood NA,et al. Interpreting insect declines:seven challenges and a way forward[J]. Insect Conserv Diver,2020,13(2):103−114. doi: 10.1111/icad.12408
[3] Hallmann CA,Sorg M,Jongejans E,Siepel H,Hofland N,et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas[J]. PLoS One,2017,12(10):e0185809. doi: 10.1371/journal.pone.0185809
[4] Sánchez-Bayo F,Wyckhuys KAG. Worldwide decline of the entomofauna:a review of its drivers[J]. Biol Conserv,2019,232:8−27. doi: 10.1016/j.biocon.2019.01.020
[5] Lister BC,Garcia A. Climate-driven declines in arthropod abundance restructure a rainforest food web[J]. Proc Natl Acad Sci USA,2018,115(44):E10397−E10406.
[6] Tong ZY,Wu LY,Feng HH,Zhang M,Armbruster WS,et al. New calculations indicate that 90% of flowering plant species are animal-pollinated[J]. Natl Sci Rev,2023,10(10):nwad219. doi: 10.1093/nsr/nwad219
[7] Steffan SA,Dharampal PS,Kueneman JG,Keller A,Argueta-Guzmán MP,et al. Microbes,the 'silent third partners' of bee-angiosperm mutualisms[J]. Trends Ecol Evol,2024,39(1):65−77. doi: 10.1016/j.tree.2023.09.001
[8] Kwon Y,Lee JT,Kim HS,Jeon C,Kwak YS. Comparative tomato flower and pollinator hive microbial communities[J]. J Plant Dis Prot,2018,125(1):115−119. doi: 10.1007/s41348-017-0090-z
[9] Eisdcowitch D,Kevan PG,Lachance MA. The nectar-inhabiting yeasts and their effect on pollen germination in common milkweed,Asclepias syriaca L.[J]. Isr J Bot,1990,39(1-2):217−225.
[10] Eisikowitch D,Lachance MA,Kevan PG,Willis S,Collins-Thompson DL. The effect of the natural assemblage of microorganisms and selected strains of the yeast Metschnikowia reukaufii in controlling the germination of pollen of the common milkweed Asclepias syriaca[J]. Can J Bot,1990,68(5):1163−1165. doi: 10.1139/b90-147
[11] Christensen SM,Munkres I,Vannette RL. Nectar bacteria stimulate pollen germination and bursting to enhance microbial fitness[J]. Curr Biol,2021,31(19):4373−4380.
[12] Canto A,Herrera CM,Medrano M,Pérez R,García IM. Pollinator foraging modifies nectar sugar composition in Helleborus foetidus (Ranunculaceae):an experimental test[J]. Am J Bot,2008,95(3):315−320. doi: 10.3732/ajb.95.3.315
[13] Peay KG,Belisle M,Fukami T. Phylogenetic relatedness predicts priority effects in nectar yeast communities[J]. Proc Roy Soc B Biol Sci,2012,279(1729):749−758.
[14] Rering CC,Beck JJ,Hall GW,McCartney MM,Vannette RL. Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator[J]. New Phytol,2018,220(3):750−759. doi: 10.1111/nph.14809
[15] Yang M,Deng GC,Gong YB,Huang SQ. Nectar yeasts enhance the interaction between Clematis akebioides and its bumblebee pollinator[J]. Plant Biol,2019,21(4):732−737. doi: 10.1111/plb.12957
[16] Herrera CM,de Vega C,Canto A,Pozo MI. Yeasts in floral nectar:a quantitative survey[J]. Ann Bot,2009,103(9):1415−1423. doi: 10.1093/aob/mcp026
[17] Shade A,McManus PS,Handelsman J. Unexpected diversity during community succession in the apple flower microbiome[J]. Mbio,2013,4(2):e00602−12.
[18] Aleklett K,Hart M,Shade A. The microbial ecology of flowers:an emerging frontier in phyllosphere research[J]. Botany,2014,92(4):253−266. doi: 10.1139/cjb-2013-0166
[19] Canto A,Herrera CM,Rodriguez R. Nectar-living yeasts of a tropical host plant community:diversity and effects on community-wide floral nectar traits[J]. PeerJ,2017,5:e3517. doi: 10.7717/peerj.3517
[20] Von Arx M,Moore A,Davidowitz G,Arnold AE. Diversity and distribution of microbial communities in floral nectar of two night-blooming plants of the Sonoran desert[J]. PLoS One,2019,14(12):e0225309. doi: 10.1371/journal.pone.0225309
[21] De Vega C,Álvarez-Pérez S,Albaladejo RG,Steenhuisen SL,Lachance MA,et al. The role of plant-pollinator interactions in structuring nectar microbial communities[J]. J Ecol,2021,109(9):3379−3395. doi: 10.1111/1365-2745.13726
[22] Vorholt JA. Microbial life in the phyllosphere[J]. Nat Rev Microbiol,2012,10(12):828−840. doi: 10.1038/nrmicro2910
[23] Rebolleda Gómez M,Ashman TL. Floral organs act as environmental filters and interact with pollinators to structure the yellow monkeyflower (Mimulus guttatus) floral microbiome[J]. Mol Ecol,2019,28(23):5155−5171. doi: 10.1111/mec.15280
[24] Russell AL,Rebolleda-Gómez M,Shaible TM,Ashman TL. Movers and shakers:bumble bee foraging behavior shapes the dispersal of microbes among and within flowers[J]. Ecosphere,2019,10(5):e02714. doi: 10.1002/ecs2.2714
[25] 周肖霄,张彦文,赵骥民,李竹月. 花部微生物生态学研究进展[J]. 植物科学学报,2023,41(1):121−127. doi: 10.11913/PSJ.2095-0837.22036 Zhou XX,Zhang YW,Zhao JM,Li ZY. Advances in floral microbial ecology[J]. Plant Science Journal,2023,41(1):121−127. doi: 10.11913/PSJ.2095-0837.22036
[26] Cariñanos P,Casares-Porcel M. Urban green zones and related pollen allergy:a review. Some guidelines for designing spaces with low allergy impact[J]. Landscape Urban Plann,2011,101(3):205−214. doi: 10.1016/j.landurbplan.2011.03.006
[27] Oldenburg M,Petersen A,Baur X. Maize pollen is an important allergen in occupationally exposed workers[J]. J Occup Med Toxicol,2011,6:32. doi: 10.1186/1745-6673-6-32
[28] Abbate JL,Gladieux P,Hood ME,de Vienne DM,Antonovics J,et al. Co-occurrence among three divergent plant-castrating fungi in the same silene host species[J]. Mol Ecol,2018,27(16):3357−3370. doi: 10.1111/mec.14805
[29] Hartmann FE,Rodríguez de la Vega RC,Carpentier F,Gladieux P,Cornille A,et al. Understanding adaptation,coevolution,host specialization,and mating system in castrating anther-smut fungi by combining population and comparative genomics[J]. Annu Rev Phytopathol,2019,57:431−457. doi: 10.1146/annurev-phyto-082718-095947
[30] Alexander HM. Epidemiology of anther-smut infection of Silene alba caused by Ustilago violacea:patterns of spore deposition and disease incidence[J]. J Ecol,1990,78(1):166−179. doi: 10.2307/2261043
[31] Jennersten O. Insect dispersal of fungal disease:effects of Ustilago infection on pollinator attraction in Viscaria vulgaris[J]. Oikos,1988,51(2):163−170. doi: 10.2307/3565638
[32] Curran HR,Roets F,Dreyer LL. Anther-smut fungal infection of South African Oxalis species:spatial distribution patterns and impacts on host fecundity[J]. South Afr J Bot,2009,75(4):807−815. doi: 10.1016/j.sajb.2009.08.004
[33] Ambika Manirajan B,Ratering S,Rusch V,Schwiertz A,Geissler-Plaum R,et al. Bacterial microbiota associated with flower pollen is influenced by pollination type,and shows a high degree of diversity and species-specificity[J]. Environ Microbiol,2016,18(12):5161−5174. doi: 10.1111/1462-2920.13524
[34] Fang Q,Chen YZ,Huang SQ. Generalist passerine pollination of a winter-flowering fruit tree in central China[J]. Ann Bot,2012,109(2):379−384. doi: 10.1093/aob/mcr293
[35] 蔡平,包立军,相入丽,张璐. 中国枇杷主要病害发生规律及综合防治[J]. 中国南方果树,2005,34(3):47−50. [36] 王继廉,李玉花. 枇杷病虫害防治要点[J]. 云南农业,2016(1):37−38. [37] Pozo MI,Herrera CM,Bazaga P. Species richness of yeast communities in floral nectar of southern Spanish plants[J]. Microbiol Ecol,2011,61(1):82−91. doi: 10.1007/s00248-010-9682-x
[38] Pozo MI,Lachance MA,Herrera CM. Nectar yeasts of two southern Spanish plants:the roles of immigration and physiological traits in community assembly[J]. FEMS Microbiol Ecol,2012,80(2):281−293. doi: 10.1111/j.1574-6941.2011.01286.x
[39] Smessaert J,van Geel M,Verreth C,Crauwels S,Honnay O,et al. Temporal and spatial variation in bacterial communities of "Jonagold" apple (Malus×domestica Borkh.) and "Conference" pear (Pyrus communis L.) floral nectar[J]. MicrobiologyOpen,2019,8(12):e918. doi: 10.1002/mbo3.918
[40] Rering CC,Rudolph AB,Li QB,Read QD,Muñoz PR,et al. A quantitative survey of the blueberry (Vaccinium spp.) culturable nectar microbiome:variation between cultivars,locations,and farm management approaches[J]. FEMS Microbiol Ecol,2024,100(3):fiae020. doi: 10.1093/femsec/fiae020
[41] Pozo MI,Herrera CM,Alonso C. Spatial and temporal distribution patterns of nectar-inhabiting yeasts:how different floral microenvironments arise in winter-blooming Helleborus foetidus[J]. Fungal Ecol,2014,11:173−180. doi: 10.1016/j.funeco.2014.06.007
[42] Toju H,Vannette RL,Gauthier MPL,Dhami MK,Fukami T. Priority effects can persist across floral generations in nectar microbial metacommunities[J]. Oikos,2018,127(3):345−352. doi: 10.1111/oik.04243
[43] Zemenick AT,Rosenheim JA,Vannette RL. Legitimate visitors and nectar robbers of Aquilegia formosa have different effects on nectar bacterial communities[J]. Ecosphere,2018,9(10):e02459. doi: 10.1002/ecs2.2459
[44] Vokou D,Vareli K,Zarali E,Karamanoli K,Constantinidou HIA,et al. Exploring biodiversity in the bacterial community of the mediterranean phyllosphere and its relationship with airborne bacteria[J]. Microb Ecol,2012,64(3):714−724. doi: 10.1007/s00248-012-0053-7
[45] Herrera CM,Canto A,Pozo MI,Bazaga P. Inhospitable sweetness:nectar filtering of pollinator-borne inocula leads to impoverished,phylogenetically clustered yeast communities[J]. Proc Roy Soc B Biol Sci,2010,277(1682):747−754.
[46] Junker RR,Keller A. Microhabitat heterogeneity across leaves and flower organs promotes bacterial diversity[J]. FEMS Microbiol Ecol,2015,91(9):fiv097. doi: 10.1093/femsec/fiv097
[47] Kosenko VN. Contributions to the pollen morphology and taxonomy of the Liliaceae[J]. Grana,1999,38(1):20−30. doi: 10.1080/001731300750044672
[48] Debray R,Herbert RA,Jaffe AL,Crits-Christoph A,Power ME,Koskella B. Priority effects in microbiome assembly[J]. Nat Rev Microbiol,2022,20(2):109−121. doi: 10.1038/s41579-021-00604-w
[49] Dhami MK,Hartwig T,Fukami T. Genetic basis of priority effects:insights from nectar yeast[J]. Proc Roy Soc B Biol Sci,2016,283(1840):20161455.
[50] Wilson M,Lindow SE. Coexistence among epiphytic bacterial populations mediated through nutritional resource partitioning[J]. Appl Environ Microbiol,1994,60(12):4468−4477. doi: 10.1128/aem.60.12.4468-4477.1994
[51] Belisle M,Peay KG,Fukami T. Flowers as islands:spatial distribution of nectar-inhabiting microfungi among plants of Mimulus aurantiacus,a Hummingbird-pollinated shrub[J]. Microb Ecol,2012,63(4):711−718. doi: 10.1007/s00248-011-9975-8
[52] Hendry TA,Ligon RA,Besler KR,Fay RL,Smee MR. Visual detection and avoidance of pathogenic bacteria by Aphids[J]. Curr Biol,2018,28(19):3158−3164. e4.
[53] Rering CC,Vannette RL,Schaeffer RN,Beck JJ. Microbial co-occurrence in floral nectar affects metabolites and attractiveness to a generalist pollinator[J]. J Chem Ecol,2020,46(8):659−667. doi: 10.1007/s10886-020-01169-3
[54] Russell AL,Ashman TL. Associative learning of flowers by generalist bumble bees can be mediated by microbes on the petals[J]. Behav Ecol,2019,30(3):746−755. doi: 10.1093/beheco/arz011
[55] Schaeffer RN,Rering CC,Maalouf I,Beck JJ,Vannette RL. Microbial metabolites elicit distinct olfactory and gustatory preferences in bumblebees[J]. Biol Lett,2019,15(7):20190132. doi: 10.1098/rsbl.2019.0132
[56] Morris MM,Frixione NJ,Burkert AC,Dinsdale EA,Vannette RL. Microbial abundance,composition,and function in nectar are shaped by flower visitor identity[J]. FEMS Microbiol Ecol,2020,96(3):fiaa003. doi: 10.1093/femsec/fiaa003
[57] Allard SM,Ottesen AR,Brown EW,Micallef SA. Insect exclusion limits variation in bacterial microbiomes of tomato flowers and fruit[J]. J Appl Microbiol,2018,125(6):1749−1760. doi: 10.1111/jam.14087



 下载:
下载: