Research progress in uptake and metabolism of foliar urea in plants
-
摘要:
叶面喷施尿素是农业生产中节肥增效和增产提质的措施之一。叶片吸收和利用尿素的过程受叶片特性、植株生长环境和体内碳氮代谢等因素影响。目前叶面肥的相关研究绝大部分仍集中在应用效果层面,而忽视了对作物叶片吸收利用尿素的过程及其调控机制的深入解析,限制了氮肥利用率和作物品质的进一步提高。本文从叶片的组织结构入手,系统综述了叶片喷施尿素后的吸收过程,涉及的关键基因和跨膜转运尿素的生理分子调控机制,以期为揭示叶面肥的高效吸收利用机理提供研究基础,并为实现节肥增效提质的农业绿色发展提供理论支撑。
Abstract:Foliar fertilization is a popular strategy used to reduce fertilizer input and improve nitrogen use efficiency, crop yield, and quality. The effectiveness of foliar application and the efficient assimilation of urea by leaves are influenced by various factors, including foliar structure, environmental conditions, and carbon-nitrogen metabolism. However, most existing studies have focused on the impacts of foliar fertilizer application, with a comprehensive understanding of the mechanisms and regulation of foliar nutrient absorption and utilization still lacking. This knowledge gap limits further improvement of nitrogen-use efficiency and crop quality. In this review, we present a systematic summary of recent advancements in leaf anatomy structure, physiological and molecular mechanisms governing urea uptake and transport, as well as the identification of key genes involved and underlying mechanisms and regulatory networks of foliar urea uptake and metabolism. This review should help inform further research, potentially guiding strategies to promote sustainable agriculture, reduce fertilizer input, and enhance efficiency and food quality.
-
Keywords:
- Leaf /
- Uptake /
- Nutrients transporter /
- Urea
-
能源安全是世界各国经济可持续发展的保障。鉴于传统化石能源日趋短缺和过度消耗造成的环境污染等,开发可再生绿色能源已成为全球关注的议题。生物质是可再生、可持续的生物能源重要原材料。续随子(Euphorbia lathyris L.)作为一种能源油料植物,具有较高的开发潜力和价值。续随子是大戟科大戟属的草本植物,在我国分布范围广泛,于江苏、浙江、福建、湖南、湖北、广西、四川等10余个省均有分布[1]。生长期一般为1~2年,且具有耐旱、喜温暖湿润、喜光照、怕水涝等特点[2]。种子油类似于石油的碳氢化合物,经过适当加工处理可制备生物燃油,具有良好的燃烧性能[3]。续随子种子油含量高达45%~60%,脂肪酸成分包括棕榈酸(16∶0)、硬脂酸(18∶0)、油酸(18∶1)、亚油酸(18∶2)、亚麻酸(18∶3)等,其中油酸含量高达总油脂含量的85%以上,棕榈酸和亚油酸分别约为8%,硬脂酸和亚麻酸分别约为2.5%[4, 5]。续随子是所有经过检测的油料植物中油酸含量最高的作物[6]。
WRI1是一种AP2/EREBP型转录因子,也是调控植物油脂合成的关键因子。这类转录因子均具有两个典型的AP2保守结构域[7],其中一个相对保守,大约60个氨基酸,另一个结构域保守性较差,且氨基酸数目较多。AP2保守结构域的末端有许多Ser和Thr氨基酸残基,C端有一个酸性结构域。如果去除C端的酸性结构域,转录活性将被阻止[8]。目前已经在多种植物中发现WRI1与体内油脂生物合成相关,主要是通过激活糖酵解及脂肪酸合成过程中的相关基因,从而促进油脂的合成[9]。WRI1可激活许多参与质体中脂肪酸从头合成的基因,包括编码质体型丙酮酸激酶(Pl-PKb1)、乙酰辅酶a羧化酶(BCCP2)、酰基载体蛋白(ACP1)和酮酰酰基载体蛋白合成酶(KAS1)的基因[10]。
在玉米(Zea mays L.)中过表达WRI1基因,可使种子含油量提高48%,且植物生长和产量并未受影响[11]。在拟南芥(Arabidopsis thaliana (L.) Heynh.)中异源表达油菜(Brassica napus L.)WRI1基因,种子中油脂含量提高了10%~40%[12]。在本氏烟草(Nicotiana benthamiana L.)中瞬时表达向日葵(Helianthus annuus L.)HaWRI1可促进其叶片油脂的大量积累[13]。在油菜和大豆(Glycine max (L.) Merr.)[14]等植物中也克隆出了WRI1基因。然而,有关续随子WRI1基因的研究还未见报道。
本研究基于全基因组序列,鉴定续随子的WRI1蛋白,对续随子ElWRI1基因的表达模式进行分析,进一步构建表达载体,并在本氏烟草叶片中进行瞬时异源表达,检测转基因叶片组织的总油脂含量及脂肪酸成分变化。研究结果旨在为全面解析续随子ElWRI1调控油脂生物合成的分子机制提供参考,并为其他植物油脂代谢遗传修饰途径研究提供新思路。
1. 材料与方法
1.1 材料
本研究所用的续随子种质材料来自山西农业大学林学院苗圃。实验采集续随子的根、茎、叶、花以及不同发育时期(分别为花后15(S1)、30(S2)、45 d(S3))的种子作为研究材料。
1.2 方法
1.2.1 ElWRI1蛋白的生物信息学分析
以从拟南芥数据库(TAIR-Home Page)获得的AtWRI1蛋白(AT3G54320)序列为模板,对续随子基因组(https://doi.org/10.6084/m9.figshare.14909913.v1.)进行BLASTP分析,鉴定获得11种长度不同的续随子ElWRI1蛋白。
使用ProParam(http://expasy.org/tools/protparam.html)软件预测ElWRI1蛋白的理论等电点、不稳定系数和疏水性等理化性质。运用MEGA 7.0软件对续随子WRI1蛋白和其他物种已知功能的WRI1蛋白序列构建系统进化树。用ProtFun(http://www.cbs.dtu.dk/services/ ProtFun/)在线软件预测蛋白质的功能分类,用SMART(http://smart.embl-heidelberg.de/)在线工具对续随子WRI1蛋白的功能结构域进行分析。利用PRALINE(http://www.ibi.vu.nl/programs/pralinewww/)在线工具对续随子和拟南芥的WRI1蛋白进行氨基酸序列多重比对分析。
1.2.2 种子不同发育时期续随子ElWRI1的表达分析
依据续随子种子发育进程,划分为3个时期(分别为花后15、30及45 d)进行取样,提取总RNA,构建cDNA文库,测序获得续随子发育种子的转录组数据(未发表数据)。依据注释的ElWRI1序列,计算各个ElWRI1成员的表达量(FPKM),绘制表达热图。
1.2.3 ElWRI1-8的基因克隆
使用TRNzol试剂盒(天根,北京)提取续随子根、茎、叶、花和3个不同发育时期种子的总RNA,反转录合成cDNA,以此为模板,特异性扩增ElWRI1-8的ORF序列。ElWRI1-8的全长 cDNA 用特异性引物扩增(ElWRI1-8-F:5'-GCTCCTCTTCATCCTCTTCTTC -3';ElWRI1-8-R:5'-ATGCCTGGTAACTCCTCTGTAA-3')。随后,使用 EasyPfu DNA 聚合酶通过RT-PCR将 cDNA用于扩增 ORF。将扩增子分别克隆到pGEM T-easy载体中。将阳性菌株送往安徽通用生物测序,测序结果与序列完全匹配。
1.2.4 ElWRI1-8表达载体的构建及瞬时表达
使用具有 Xba Ⅰ(5'-末端)和 Kpn Ⅰ(3'-末端)位点的基因特异性引物(F:5'-GCTCTAGAATGGGAAACAAGGAAACAATGG -3'; R:5'-CCCCCGGGCTACAACACTCTCAGTTGAAGAT-3'),从相应的克隆载体中分别扩增ElWRI1-8。然后将其插入 pCAMBIA1303载体中。再将含有ElWRI1-8的载体通过冻融方法转化到根癌农杆菌GV3101中。参考霍琳等[15]的方法侵染烟草叶片并取样。
1.2.5 脂肪酸甲酯和总油脂测定
参考任国鹏等[16]的方法提取转基因烟草叶片的总油脂和脂肪酸。参考王计平等[17]及李璐[18]的方法,使用安捷伦7890B气象色谱仪(Agilent,美国)测定烟草叶片脂肪酸各组分含量,脂肪酸含量利用安捷伦软件进行分析。
2. 结果与分析
2.1 ElWRI1的全基因组鉴定及蛋白保守基序分析
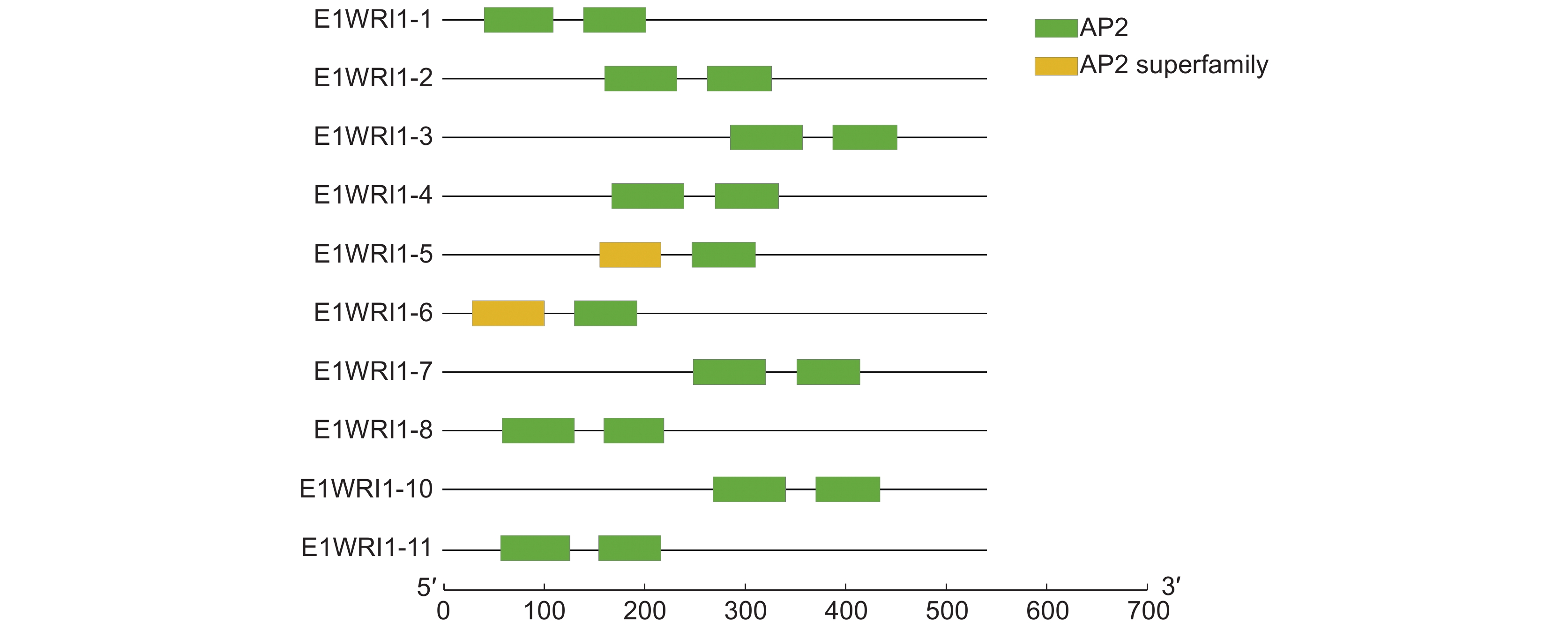
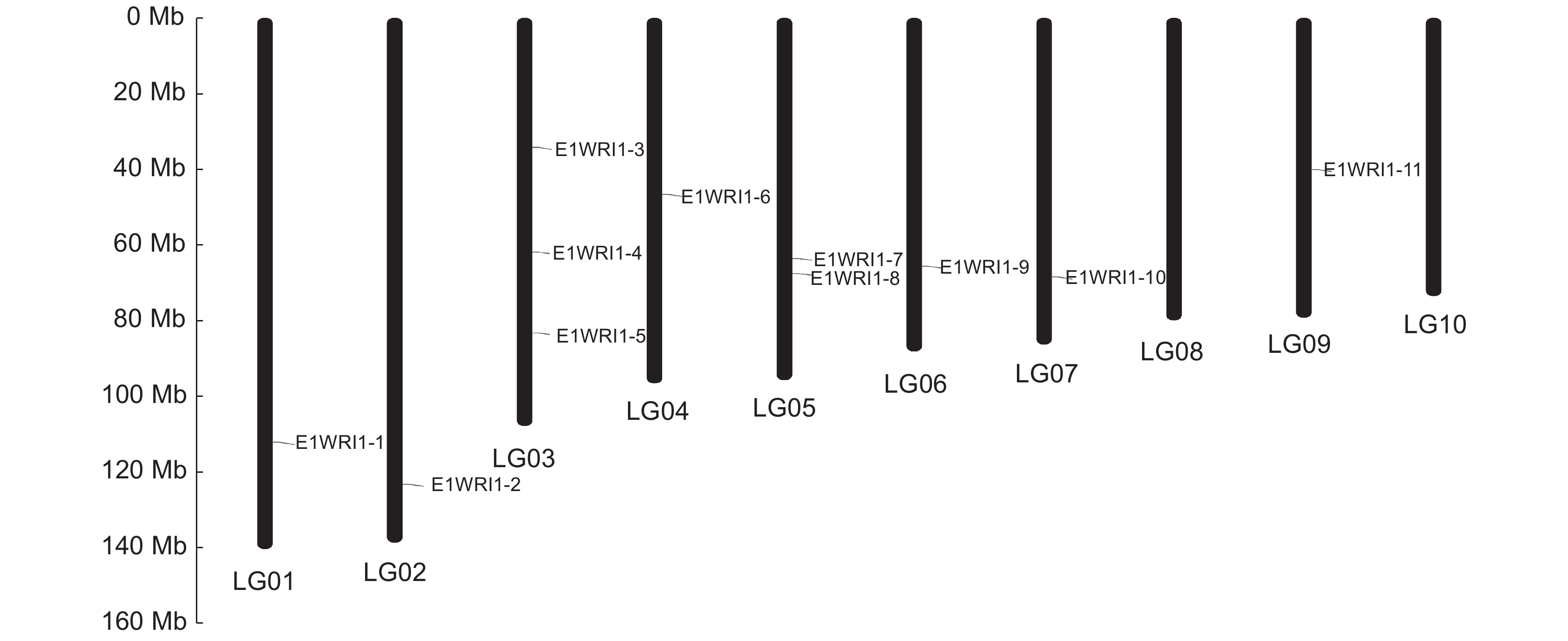
应用 NCBI 网站的Genome Date Viewer工具,获得续随子WRI1各基因的染色体信息(图1)。续随子WRI1基因分布于 1~7和9号染色体上,分别命名为 WRI1-1~WRI1-11。
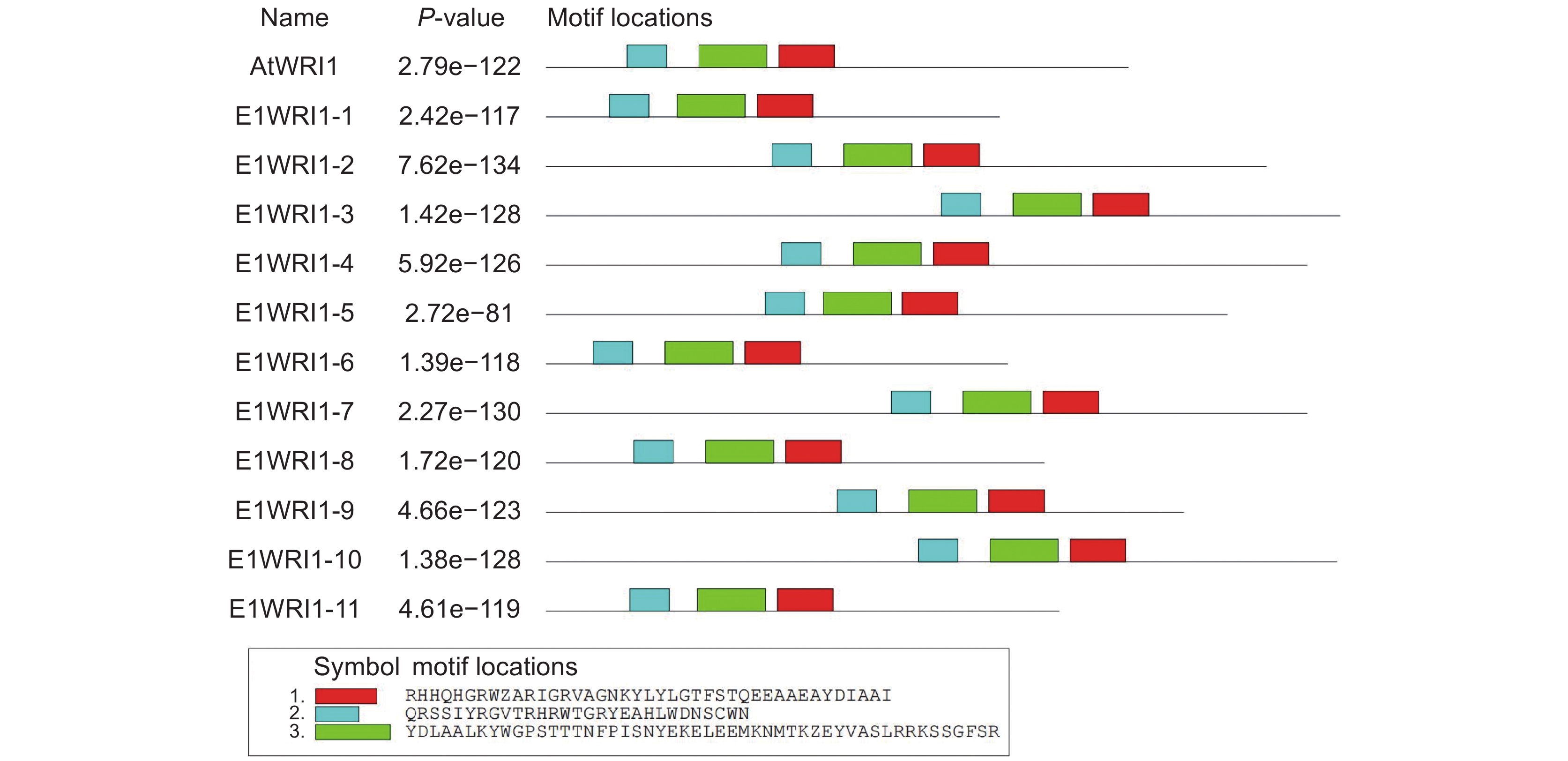
通过CDD鉴定WRI1的功能结构域,结果如图2所示。11个WRI1蛋白均含有两个AP2保守结构域,均属于AP2/EREBP家族。续随子ElWRI1蛋白保守基序分析结果如图3所示,ElWRI1蛋白具有相似的基序排列,均包含3个motif。这也表明这些蛋白质具有类似的功能。
2.2 续随子WRI1基因结构分析
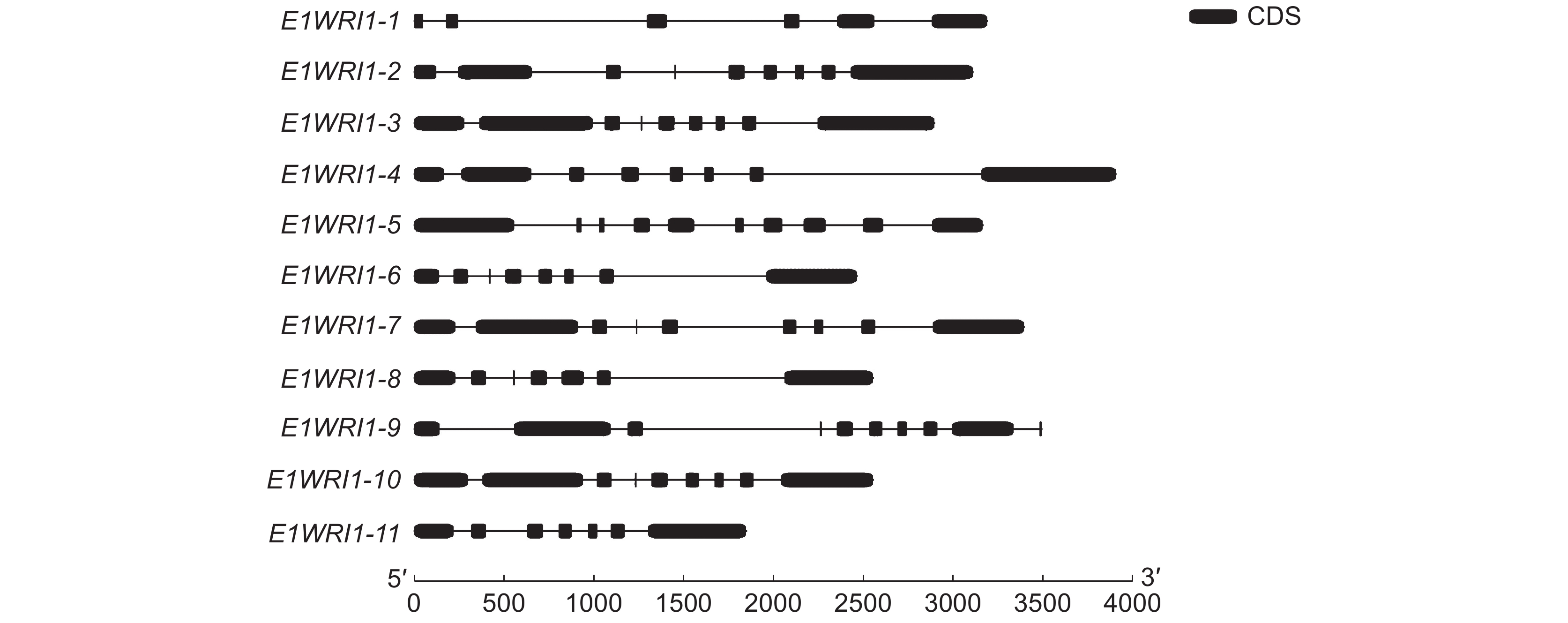
利用 GSDS 2.0网站分析WRI1的基因结构,结果显示(图4),WRI1-4序列最长,WRI1-11序列最短。此外,WRI1-6、WRI1-8、WRI1-11基因结构中包含7个外显子和6个内含子,而WRI1-2、WRI1-3、WRI1-7和WRI1-9基因均有9个外显子和8个内含子,WRI1-4和WRI1-10都有8个外显子和7个内含子。内含子和外显子数量最多的是WRI1-5,有10个外显子和9个内含子。内含子和外显子数量最少的是WRI1-1,有5个外显子和4个内含子。
2.3 续随子WRI1蛋白的理化性质分析
续随子WRI1蛋白的基本理化性质见附表1
1 ,WRI1蛋白含334 ~ 646个氨基酸,相对分子量为 38.25 ~ 71.97 kD,其中WRI1-3氨基酸最多,相对分子量最大,WRI1-1的氨基酸最少,相对分子量最小。WRI1-1、WRI1-6、WRI1-8和WRI1-11的理论等电点大于7,为碱性蛋白,其余蛋白的等电点均小于7,为酸性蛋白。11个ElWRI1转录因子均为不稳定蛋白。亲水性指数均为负值,表明ElWRI1蛋白为亲水性蛋白。所有WRI1蛋白均无跨膜结构,且预测均定位于细胞核。此外,WRI1-3、WRI1-6、WRI1-8、WRI1-10和WRI1-11存在信号肽序列,为分泌蛋白。2.4 续随子ElWRI1蛋白的高级结构预测
续随子ElWRI1蛋白二级结构预测结果见附表2
1 。WRI1蛋白的二级结构由无规则卷曲、α螺旋、延伸链和β-折叠组成。每个WRI1蛋白二级结构的主体相似,但各个组成结构的比例存在一定差异,均为α螺旋和无规则卷曲所占比例最高,其中β折叠所占比例最低。2.5 续随子ElWRI1蛋白多序列比对和系统进化分析
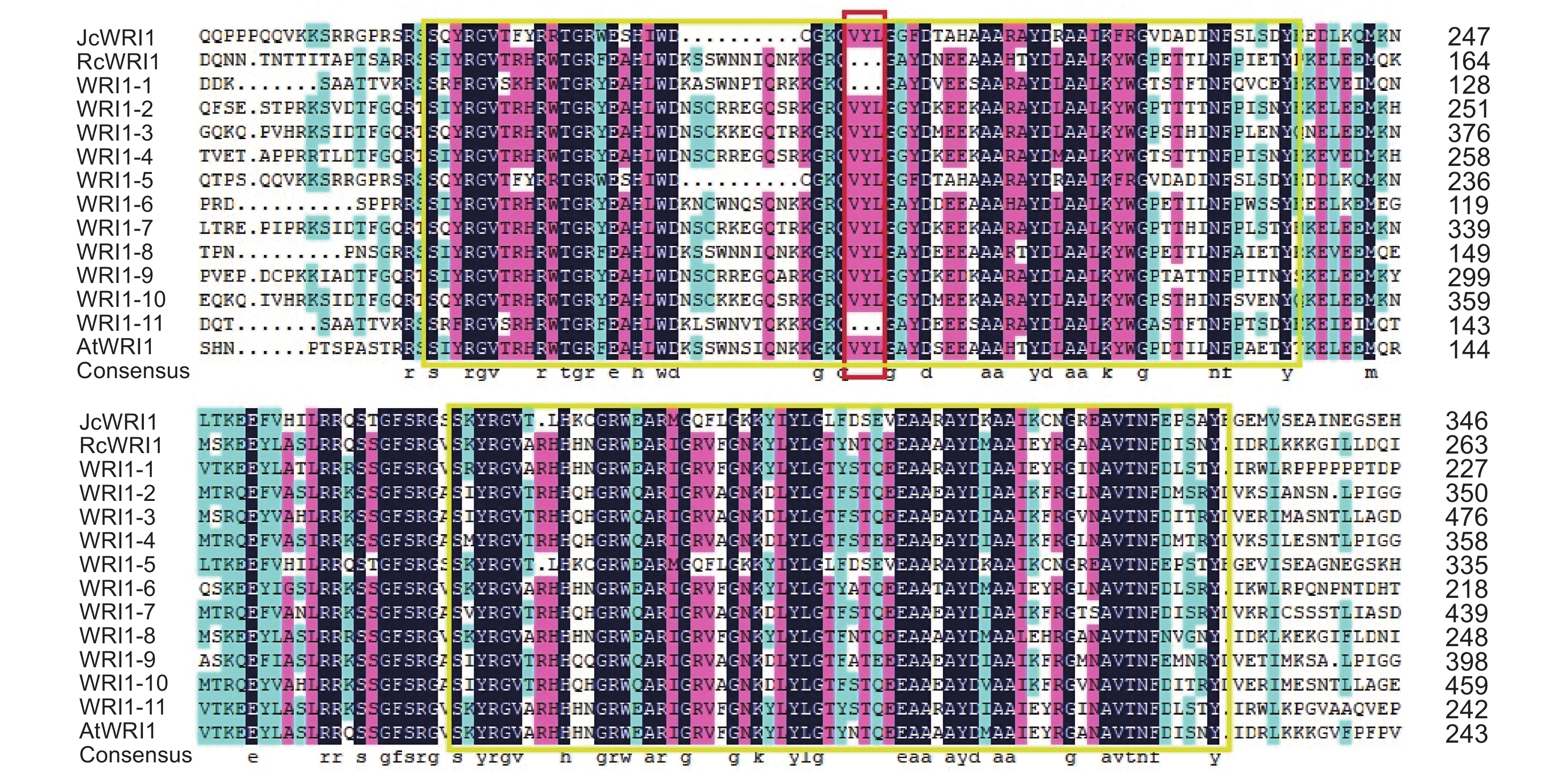
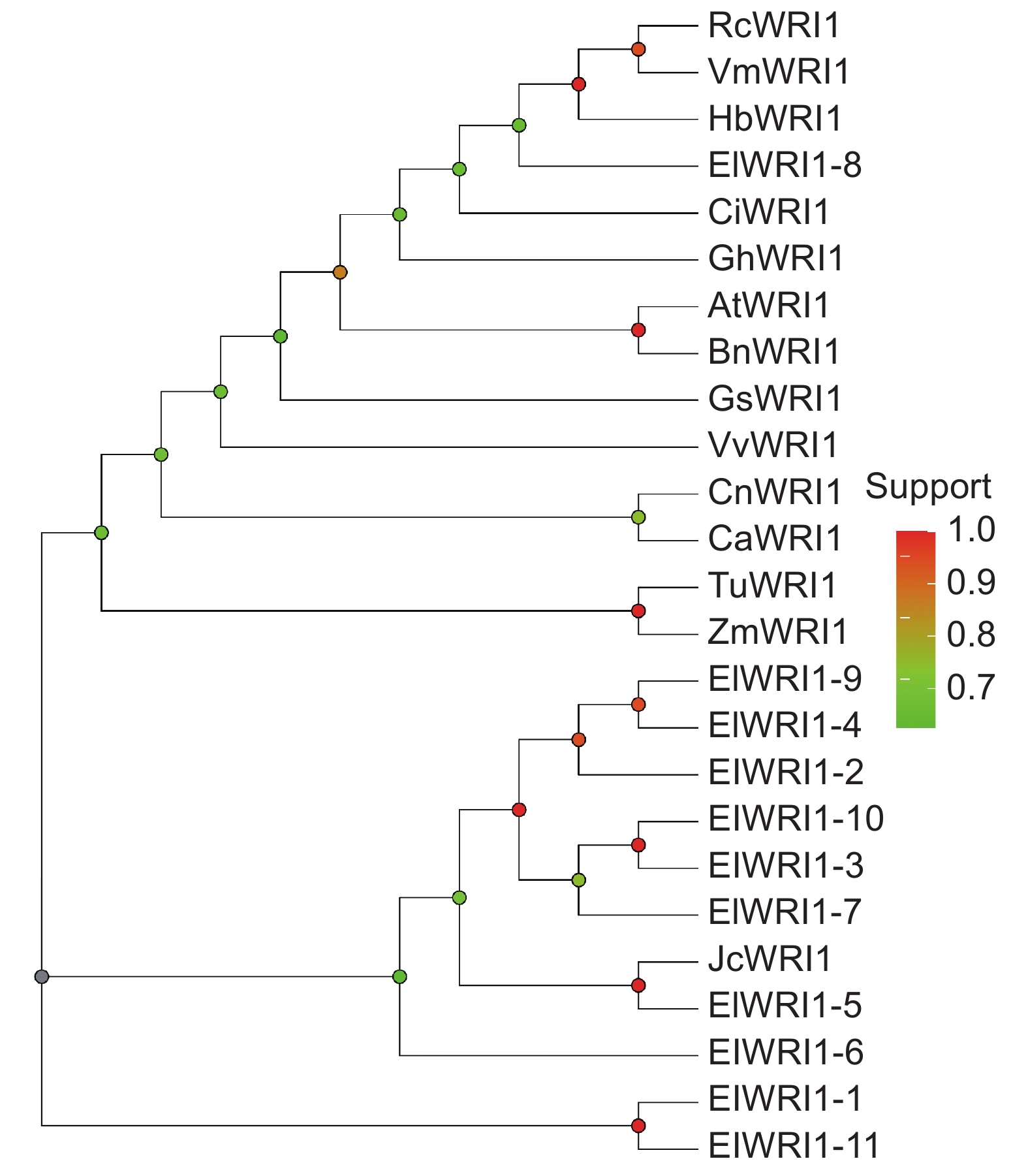
选择同属于大戟科的蓖麻(Ricinus communis L.)RcWRI1和麻风树(Jatropha curcas L.)JcWRI1以及模式植物拟南芥AtWRI1为参考序列,利用DNAman软件对续随子ElWRI1蛋白进行多序列比对分析,结果如图5所示。续随子WRI1与麻风树JcWRI1、蓖麻RcWRI1和拟南芥AtWRI1相似,均包含2个相对保守的AP2/EREBP结构域区域。除ElWRI1-1和ElWRI1-11之外,其余ElWRI1蛋白序列均具有“VYL”氨基酸结构。
系统发育分析结果显示(图6),ElWRI1-2、ElWRI1-3、ElWRI1-4、ElWRI1-9、ElWRI1-7和ElWRI1-10的亲缘关系较近,聚为一支;ElWRI1-5与麻风树JcWRI1聚为一支;ElWRI1-8则与蓖麻RcWRI1、巴西橡胶树(Hevea brasiliensis(Willd. ex A. Juss.)Muell. Arg.)HbWRI1、皱果桐(Vernicia montana Lour.)VmWRI1聚在一起。
![]() 图 6 不同物种WRI1蛋白的系统发育分析RcWRI1:蓖麻 (NP_001310691.1);AtWRI1:拟南芥 (OAP01705.1);JcWRI1:麻风树 (AIR74897.1);HbWRI1:巴西橡胶树 (XP_021674385.1);ZmWRI1:玉米 (ONM19989.1);BnWRI1:甘蓝型油菜 (ADO16346.1);CsWRI1:大豆 (KHN10130.1);VvWRI1:葡萄 (XP_010659009.1); CaWARI:辣椒(KAF3665339.1); CiWRI1:山核桃 (QQP23243.1); CnWRI1: 椰子 (KAG1365549.1);TuWRI1:乌拉图小麦 (EMS52893.1); GhWRI1:陆地棉(NP_001314053.1);VmWRI1:皱果桐(APQ47387.1)。Figure 6. Phylogenetic analysis of ElWRI1 among different plant speciesRcWRI1: Ricinus communis (NP_001310691.1); AtWRI1: Arabidopsis thaliana (OAP01705.1); JcWRI1: Jatropha curcas (AIR74897.1); HbWRI1: Hevea brasiliensis (XP_021674385.1); ZmWRI1: Zea mays (ONM19989.1); BnWRI1: Brassica napus (ADO16346.1); CsWRI1: Clycine soja (KHN10130.1); VvWRI1: Vitis vinifera (XP_010659009.1); CaWARI: Capsicum annuum (KAF3665339.1); CiWRI1: Carya illinoinensis (QQP23243.1); CnWRI1: Cocos nucifera (KAG1365549.1); TuWRI1: Triticum urartu (EMS52893.1); GhWRI1: Gossypium hirsutum (NP_001314053.1); VmWRI1: Vernicia montana (APQ47387.1).
图 6 不同物种WRI1蛋白的系统发育分析RcWRI1:蓖麻 (NP_001310691.1);AtWRI1:拟南芥 (OAP01705.1);JcWRI1:麻风树 (AIR74897.1);HbWRI1:巴西橡胶树 (XP_021674385.1);ZmWRI1:玉米 (ONM19989.1);BnWRI1:甘蓝型油菜 (ADO16346.1);CsWRI1:大豆 (KHN10130.1);VvWRI1:葡萄 (XP_010659009.1); CaWARI:辣椒(KAF3665339.1); CiWRI1:山核桃 (QQP23243.1); CnWRI1: 椰子 (KAG1365549.1);TuWRI1:乌拉图小麦 (EMS52893.1); GhWRI1:陆地棉(NP_001314053.1);VmWRI1:皱果桐(APQ47387.1)。Figure 6. Phylogenetic analysis of ElWRI1 among different plant speciesRcWRI1: Ricinus communis (NP_001310691.1); AtWRI1: Arabidopsis thaliana (OAP01705.1); JcWRI1: Jatropha curcas (AIR74897.1); HbWRI1: Hevea brasiliensis (XP_021674385.1); ZmWRI1: Zea mays (ONM19989.1); BnWRI1: Brassica napus (ADO16346.1); CsWRI1: Clycine soja (KHN10130.1); VvWRI1: Vitis vinifera (XP_010659009.1); CaWARI: Capsicum annuum (KAF3665339.1); CiWRI1: Carya illinoinensis (QQP23243.1); CnWRI1: Cocos nucifera (KAG1365549.1); TuWRI1: Triticum urartu (EMS52893.1); GhWRI1: Gossypium hirsutum (NP_001314053.1); VmWRI1: Vernicia montana (APQ47387.1).2.6 续随子ElWRI1表达分析
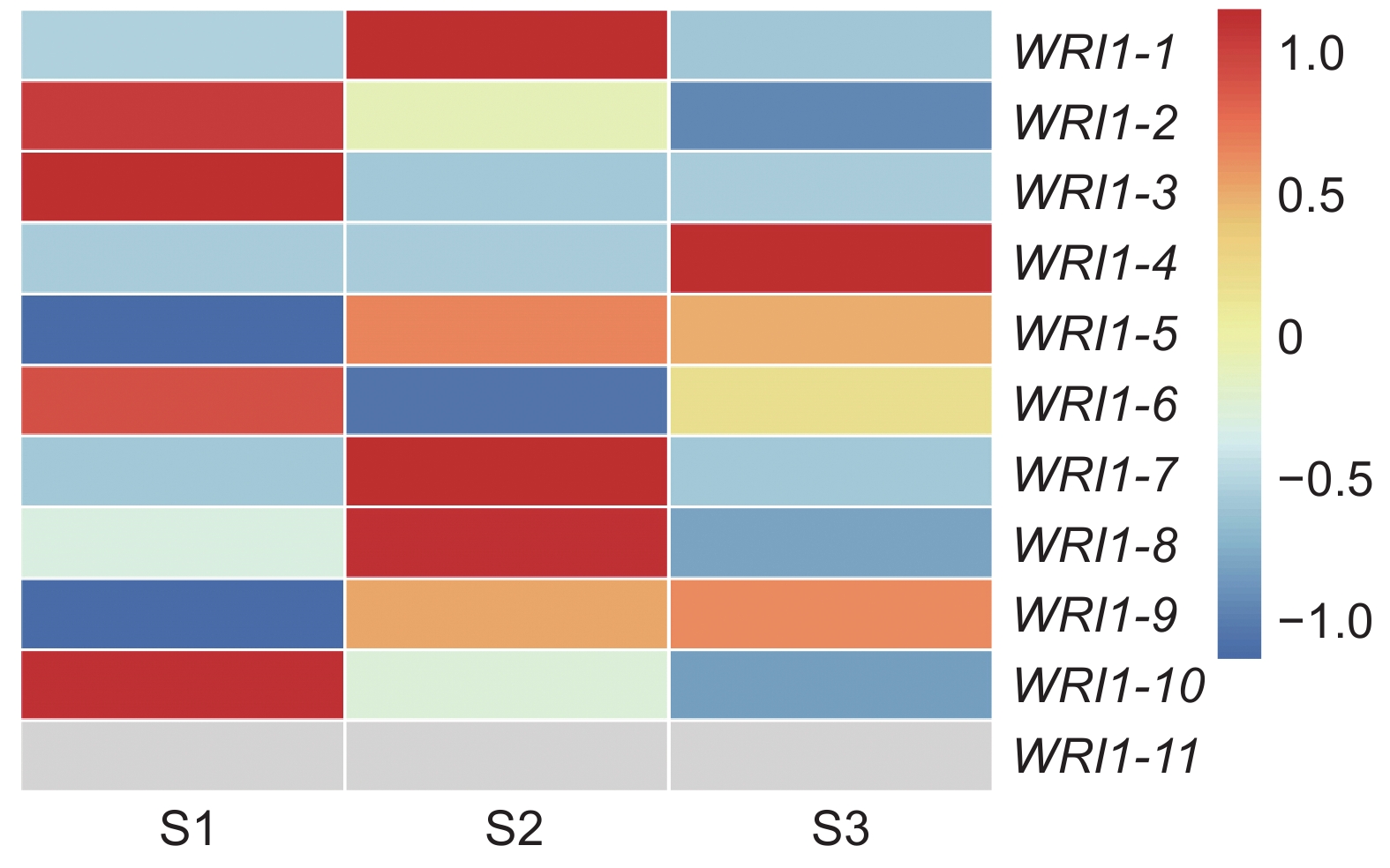
利用课题组已有的转录组数据,构建种子不同发育时期续随子ElWRI1的表达谱热图(图7)。样品来自种子发育的3个不同时期(S1、S2和S3)。除ElWRI1-5、ElWRI1-9和ElWRI1-11全程表达量偏低外,ElWRI1-2、ElWRI1-3、ElWRI1-6和ElWRI1-10在S1时期表达量最高;ElWRI1-1、ElWRI1-7和ElWRI1-8在S2时期表达量最高;而ElWRI1-4在S3时期表达量最高。
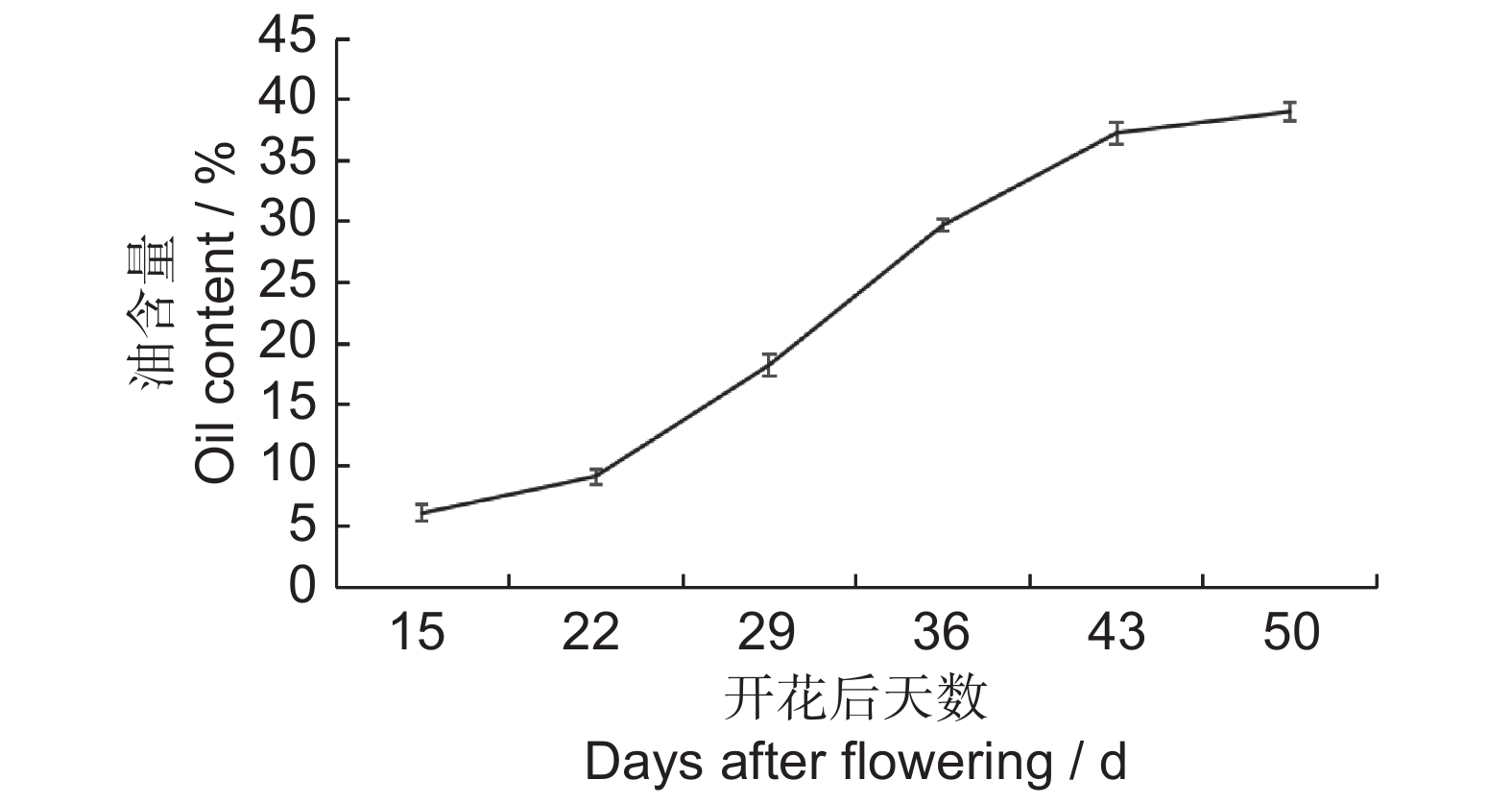
分析续随子种子不同发育时期含油率的变化(图8),发现花后22~43 d是续随子种子油脂积累的关键时期。因此,选择在S2时期表达量达到最高的WRI1-8进行下一步实验。
2.7 续随子ElWRI1-8基因表达载体的构建及烟草叶片的瞬时表达
为探究续随子ElWRI1-8基因对油脂合成的影响,以S2时期(开花后30 d)的cDNA为模板克隆续随子ElWRI1-8基因,并构建获得植物表达载体pCAMBIA1303-ElWRI1-8。
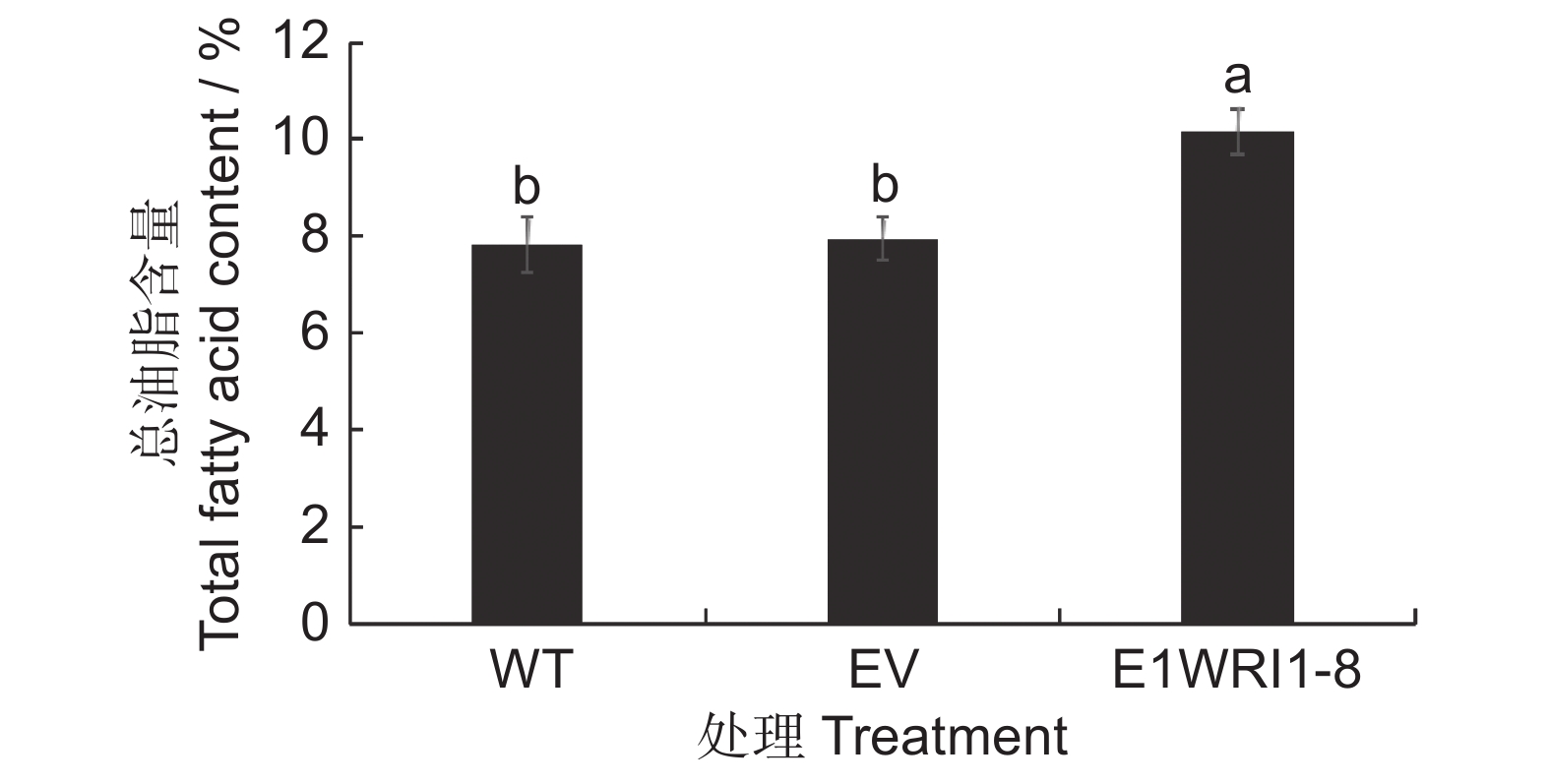
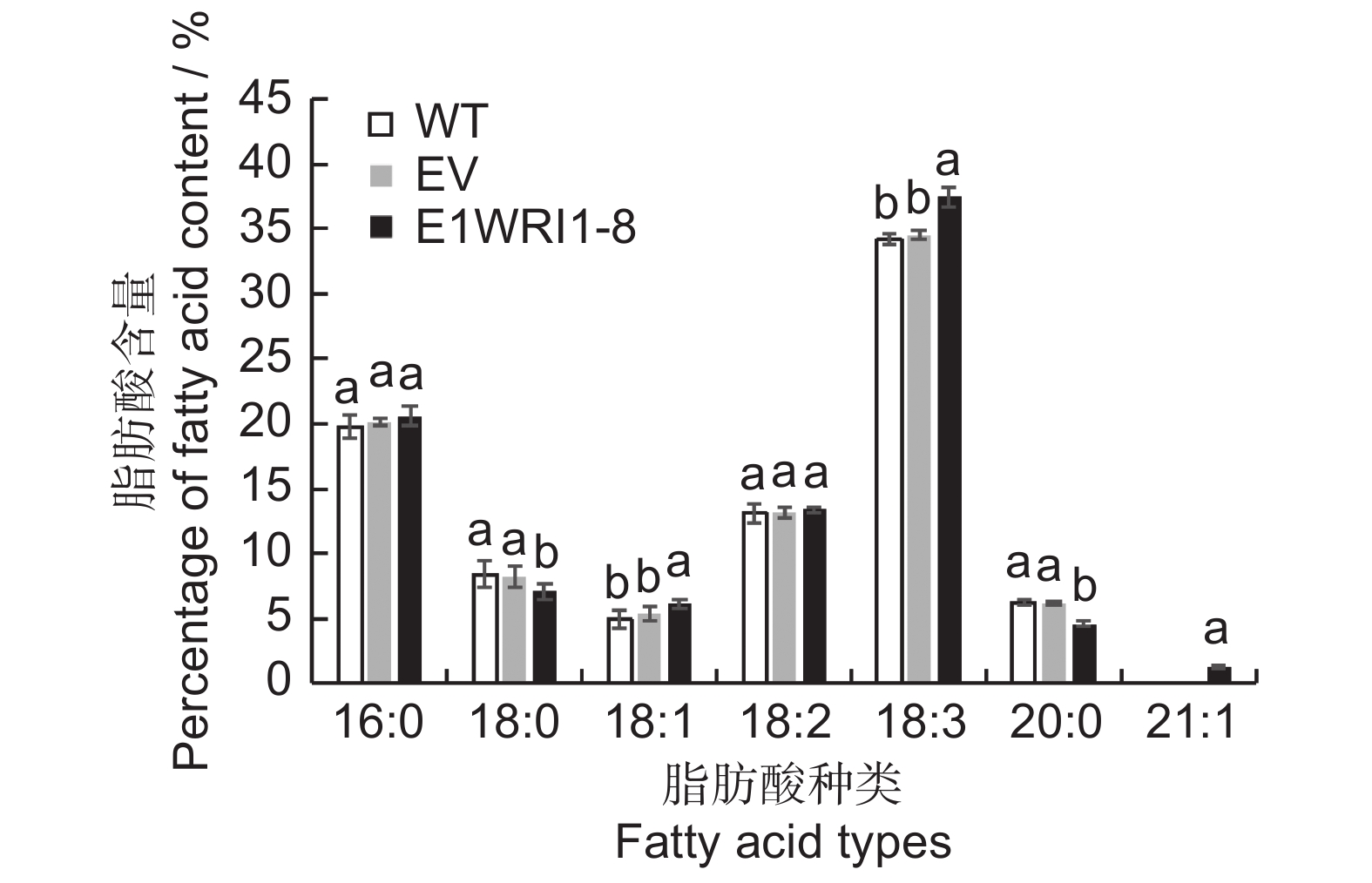
将含有目的基因及空载体质粒转入烟草叶片中进行瞬时表达,分析ElWRI1-8对烟草叶片总脂肪酸含量以及脂肪酸成分变化的影响(图9、图10)。总油脂含量检测结果如图9所示,与野生型(WT)叶片相比,转入空载体(EV)后总油脂含量几乎不变,而过表达ElWRI1-8后总脂肪酸含量增加显著,由7.83%上升至10.16%,是野生型的1.3倍。说明瞬时表达续随子ElWRI1-8基因后,可以在一定程度上提高烟草叶片的总油脂含量。脂肪酸组分测定结果显示(图10),转入基因ElWRI1-8后,脂肪酸16∶0和18∶2的变化不明显,脂肪酸18∶1和18∶3的含量比野生型及转空载有所上升,其中18∶1含量上升19.60%,18∶3含量上升8.90%。脂肪酸18∶0和20∶0的含量比野生型及转空载有所下降,前者下降14.97%,后者下降26.08%。瞬时表达后检测到一种新的长链脂肪酸22∶1,表明ElWRI1-8的表达会导致烟草叶片脂肪酸组分发生变化,且在一定程度上促进长链脂肪酸的合成。
3. 讨论
WRI1转录因子主要在油脂积累、糖酵解以及胚胎发育中起调控作用,WRI1最早在拟南芥突变体中被发现,并在随后分离克隆到AtWRI1基因。目前已在多种植物中进行了WRI1基因的克隆和表达研究[19]。
选择性剪切是调控基因表达的一种方式,至少有42%的拟南芥基因是选择性剪切的[20]。Ma等[21]研究发现,拟南芥AtWRI1有多种剪切形式,但只有剪接形式3(At3g54320.3)存在于拟南芥的多个组织和器官中。本文以AtWRI1(At3g54320.3)为索引,在续随子基因组中鉴定得到11个ElWRI1基因,并根据其在染色体上的位置分别命名为ElWRI1-1~ElWRI1-11。
多序列比对结果显示,除ElWRI1-1和ElWRI1-11之外,其余的ElWRI1蛋白序列均具有“VYL”氨基酸结构,这与An等[8]对拟南芥AtWRI1和亚麻荠CsWRI1的多序列比对结果一致。拟南芥AtWRI1第3外显子长度仅为9 bp,编码氨基酸“VYL”。这也符合An等[8]的研究结论,即大多数WRI1基因,包括AtWRI1,有7个外显子,其中第3个外显子仅包含9个核苷酸,且序列高度保守,编码WRI1蛋白的第1个AP2结构域的3个氨基酸残基(“VYL”)。Ma等[21]和Krizek等[22]的研究均表明,“VYL”中的1个或两个氨基酸的缺失,都会导致WRI1基因功能的降低或者缺失。
续随子11条ElWRI1序列都具有两个保守的AP2结构域,属AP2型转录因子。这与Riechmann等[23]提出的WRI1是一种AP2/乙烯反应元件结合蛋白(AP2/EREBP)型转录因子,这种转录因子至少有1个AP2保守结构域的结论一致。ElWRI1均为不稳定亲水蛋白,无跨膜结构,且均定位于细胞核,可能在细胞核中发挥作用。此外,ElWRI1-3、ElWRI1-6、ElWRI1-8、ElWRI1-10和ElWRI1-11存在信号肽,可能为分泌蛋白。表达分析结果显示,在续随子种子发育时期ElWRI1-8的表达量显著高于其他基因。由此推测,ElWRI1-8可能在续随子种子油脂合成积累过程中起着更为主导的调控作用。
为了进一步验证ElWRI1-8的功能,本研究通过农杆菌介导的侵染法将其转入烟草叶片中进行瞬时表达,并测定分析叶片中总脂肪酸含量以及脂肪酸组分。结果发现,与空载对照相比,转基因烟草叶片中的总脂肪酸含量显著升高,脂肪酸18∶1和18∶3的含量有所提高,脂肪酸18∶0和20∶0的含量则有所下降,此外,还检测到了对照中没有的长链脂肪酸22∶1。
综上所述,本研究鉴定获得了续随子ElWRI1-8基因,该基因具有AP2/EREBP型转录因子的典型特征,并在本氏烟草中通过异源过表达进行了功能验证。研究结果表明续随子ElWRI1-8基因具有提高植物油脂含量或改变植物脂肪酸成分的潜在应用价值。然而,过表达ElWRI1-8基因在提高烟草叶片油脂合成的同时,对淀粉、蛋白质和可溶性糖的合成是否产生影响,以及该基因主要调控哪些靶基因,还有待进一步研究。
-
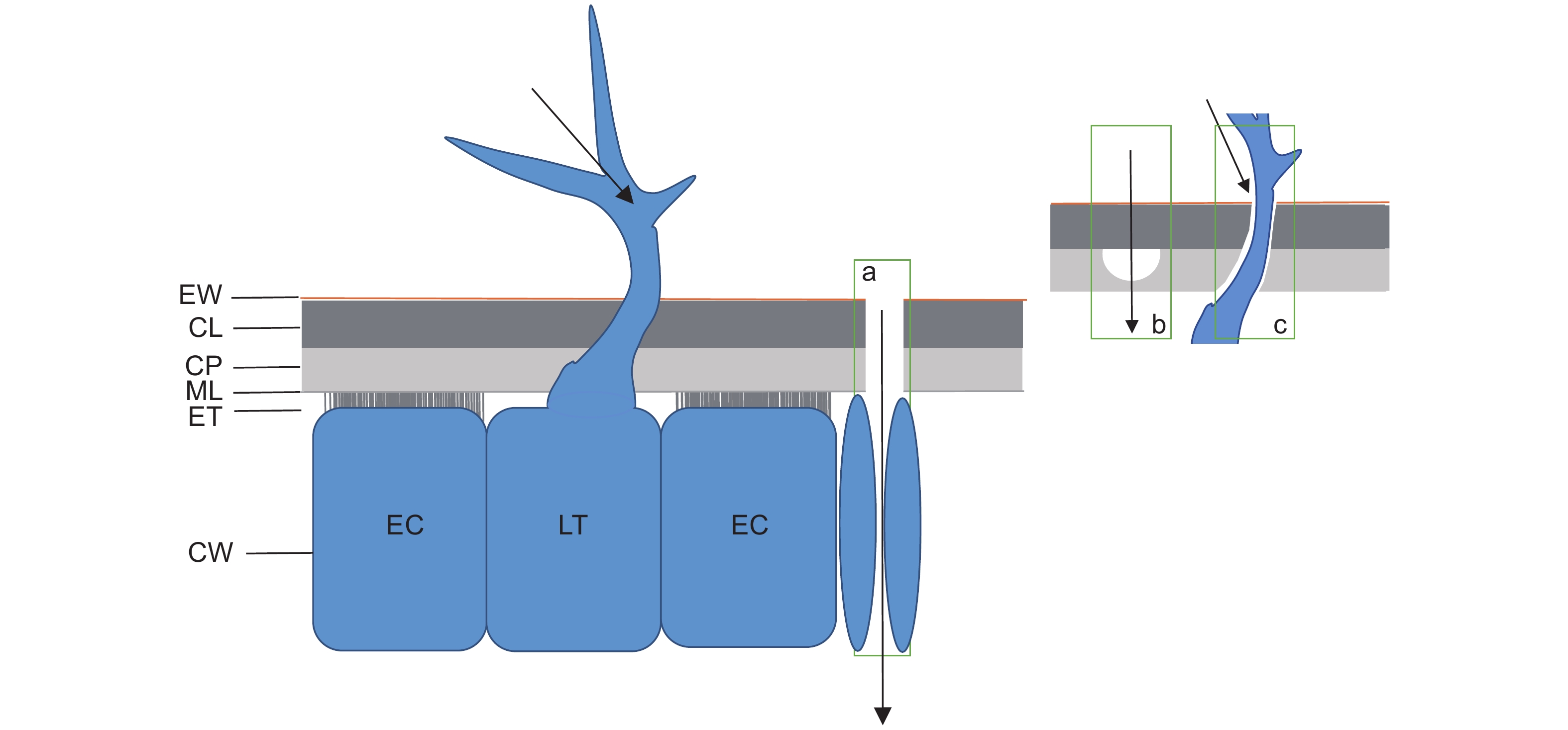
图 1 尿素进入叶片表皮细胞的途径示意图
a:尿素通过角质层的亲水小孔进入叶表;b:尿素通过叶片表面腺毛和叶片交接处缝隙进入叶表;c:尿素通过气孔进入表皮细胞和细胞间隙。EW:外角质层;CP:真角质层;CL:角质层基层;ML:中间板层和果胶层;ET:外质连丝;CW:细胞壁;EC:表皮细胞;LT:毛状体。
Figure 1. Schematic of urea pathway into leaf epidermal cells
a: Urea enters the leaf surface through hydrophilic pores in the cuticle; b: Urea enters the leaf surface through the gap between the leaf surface gland hairs and leaf junction; c: Urea enters the epidermal cells and intercellular spaces through the stomata. EW: Epicuticular waxes; CP: Cuticle proper; CL: Cuticular layer; ML: Middle lamellae and pectinaceous Layer; ET: Ectodesmata; CW: Cell wall; EC: Epidermal cells; LT: Leaf trichome.
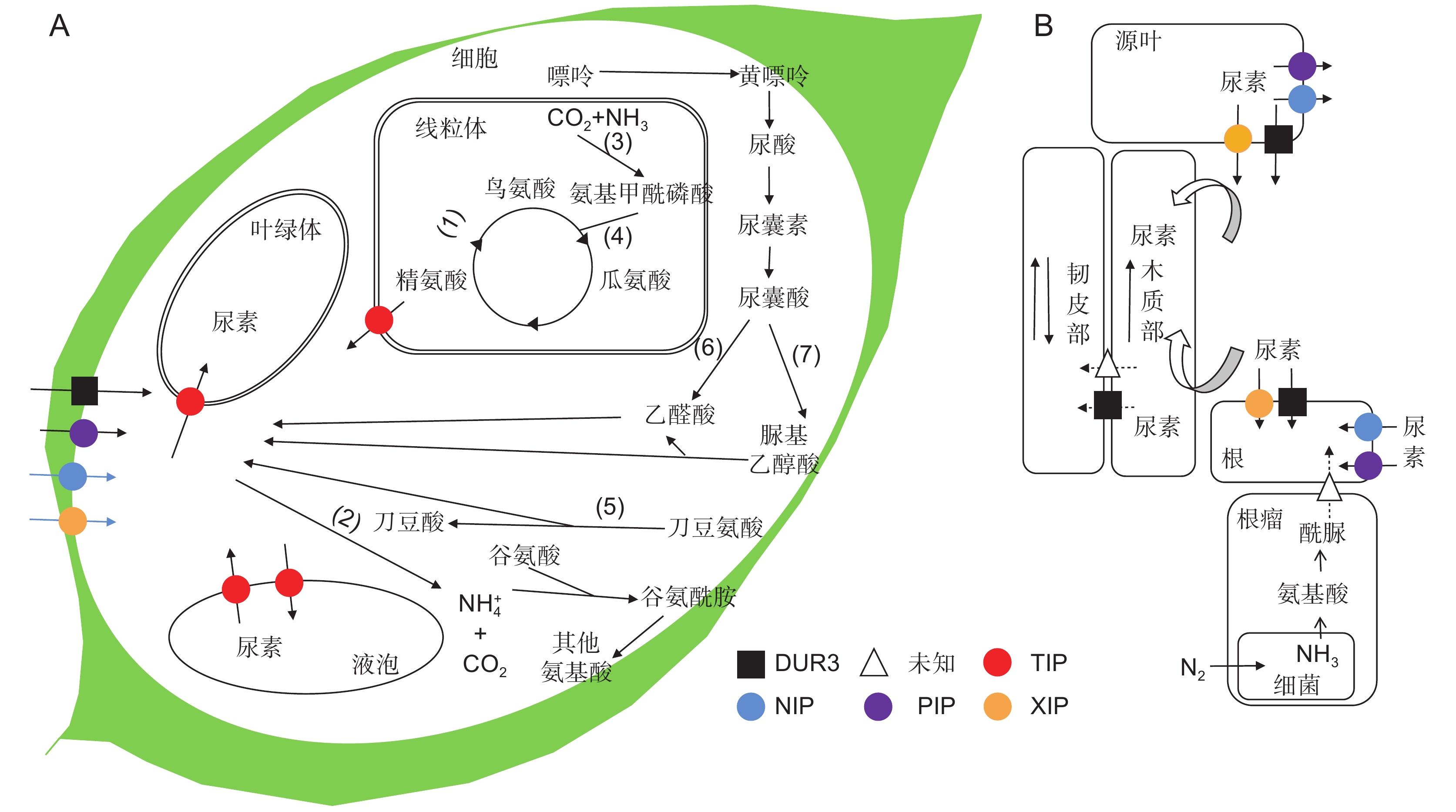
图 2 植物对尿素的吸收与代谢(参考曹凤秋等[53]和Tegeder等[57]的文献)
A:细胞中尿素的转运、分解与合成过程。植物中尿素合成和分解的酶主要包括:(1)精氨酸酶;(2)脲酶;(3)氨基甲酰磷酸合成酶;(4)鸟氨酸氨基甲酰转移酶;(5)刀豆氨酸水解酶;(6)尿囊酸脒基水解酶;(7)尿囊酸酰胺水解酶;(8)尿素裂解酶。DUR3是高亲和尿素转运蛋白;TIP、NIP、PIP和XIP亚家族的水通道蛋白介导低亲和的尿素转运。B:根、叶尿素吸收以及从根到叶的长距离运输。实线箭头表示已知途径。虚线箭头表示未知途径。
Figure 2. Urea uptake and metabolism in plants (modified according to Cao et al.[53] and Tegeder et al.[57])
A: Schematic of transport, decomposition, and synthesis of urea in cells. Urea-generating enzymes are (1) Arginase; (2) Urease; (3) Carbamoyl phosphate synthetase; (4) Ornithinetranscarbamylase; (5) Canavanine hydrolase; (6) Amidinohydrolase; (7) Allantoate amidinohydrolase; (8) Urea-lyase. DUR 3 is a high-affinity urea transporter; aquaporins of TIP, NIP, PIP, and XIP subfamilies mediate low-affinity urea transport. Schematic representation of urea uptake and translocation from root to leaves. Legend indicates transporters involved in urea transport. B: Root, leaf urea uptake, and long-distance transport from root to leaf. Solid-line arrows indicate known pathways. Dashed arrows indicate unknown pathway.
表 1 植物叶片中尿素转运体
Table 1 Urea transporters in leaves
类型
Type基因
Gene登录号
Accession No.物种
Species拉丁名
Latin name位点
Site功能
Function文献
ReferenceDUR3 AtDUR3 At5g45380 拟南芥 Arabidopsis thaliana (L.) Heynh. 细胞质膜 H + -尿素共运输
高亲和性尿素跨质膜
吸收转运;[26] OsDUR3 Os10g0580400 水稻 Oryza sativa L. 细胞质膜 尿素运输;氮转移 [27] ZmDUR3 Zm00001e030875 玉米 Zea mays L. 细胞质膜 高亲和力尿素转运 [28] PyDUR3.1 AB359179 条斑紫菜 Porphyra yezoensis Ueda – 氮缺乏高度上调 [29] PyDUR3.2 AB359180 条斑紫菜 – 氮缺乏高度上调 [29] PyDUR3.3 AB931115 条斑紫菜 – 尿素转运 [30] PIP ZmPIP1.5 Zm00001d051872 玉米 Zea mays L. 质膜 尿素转运蛋白 [31] ZmPIP1.5b AJ271796 玉米 – 尿素转运蛋白 [31] NIP ZmNIP2.1 Zm00001d018037 玉米 质膜 尿素吸收与转运;
调节液泡中尿素[32] ZmNIP2.4 Zm00001d052261 玉米 质膜 尿素吸收与转运;
调节液泡中尿素[32] OsNIP2;1 Os02g0745100 水稻 Oryza sativa L. – 尿素转运蛋白 [33] CsNIP2;1 Csa017389 黄瓜 Cucumis sativus L. – 尿素的吸收和内部运输 [34] AtNIP6.1 At1g80760 拟南芥 Arabidopsis thaliana (L.) Heynh. 质膜 尿素转运蛋白 [35] AtNIP5.1 At4g10380 拟南芥 – 低硼诱导的尿素转运 [35] TIP AtTIP1.1 At2g36830 拟南芥 液泡膜 尿素转运蛋白 [36] AtTIP1.2 At3g26520 拟南芥 液泡膜 尿素转运蛋白 [24] AtTIP1.3 At4g01470 拟南芥 – 尿素转运蛋白 [37] AtTIP2.1 At3g16240 拟南芥 液泡膜/
叶绿体膜尿素转运蛋白 [24] AtTIP4.1 At2g25810 拟南芥 液泡膜 尿素转运蛋白 [24] AtTIP5.1 At3g47440 拟南芥 线粒体膜 尿素转运蛋白 [38] NtTIPa AJ237751 拟南芥 液泡膜 调节液泡中尿素 [39] VgTIP2.1 MH824554 巨大丽穗凤梨 Vriesea gigantea Gaudich 液泡膜 调节液泡中尿素 [40] ZmTIP4.4 Zm00001d039293 玉米 Zea mays L. 液泡膜 调节液泡中尿素 [22] XIP NtXIP1.1α NP_001312796 烟草 Nicotiana tabacum L. 质膜 尿素转运蛋白 [41] NtXIP1.1β Nitab4.5_0000956g0150.1 烟草 质膜 尿素转运 [41] 表 2 不同类型植物中尿素合成与分解代谢比较
Table 2 Urea synthesis, catabolism, and degradation in different types of plants
表 3 植物叶片中铵态氮转运体
Table 3 Ammonium-nitrogen transporters in leaves
类型
Type基因
Gene登录号
Accession No.物种
Species拉丁名
Latin name位点
Site功能
Function文献
ReferencePIP VgPIP1.1 MH824552 巨大丽穗凤梨 Vriesea gigantea Gaudich – 促进NH4 + /NH3转运 [40] VgPIP1.2 MH824553 巨大丽穗凤梨 – 促进NH4 + /NH3转运 [40] AMT AtAMT1.1 AT4G13510 拟南芥 Arabidopsis thaliana (L.) Heynh. 质膜 NH4 + 转运蛋白 [62] AtAMT1.3 AT3G24300 拟南芥 质膜 NH4 + 转运蛋白 [63] AtAMT1.4 AT4G28700 拟南芥 – NH4 + 转运蛋白 [66] AtAMT1.5 AT3G24290 拟南芥 – NH4 + 转运蛋白 [64] AtAMT2.1 AT2G38290 拟南芥 质膜 高亲和NH4 + 转运蛋白 [67] BnAMT1.2 AF306518 油菜 Brassica napus L. – NH4 + 转运蛋白 [63] LeAMT1.3 NP_001234216.1 番茄 Solanum lycopersicum L. – NH4 + 转运蛋白 [65] GmAMT1.6 GLYMA_20G221400 大豆 Glycine max (L.) Merr. – NH4 + 转运蛋白 [62] GmAMT2.2 GLYMA_18G204800 大豆 – NH4 + 转运蛋白 [67] GmAMT3.1 GLYMA_05G196500 大豆 – NH4 + 转运蛋白 [62] PtrAMT3.1 Poptr1_1:175190 杨 Populus – NH4 + 转运蛋白 [64] TIP AtTIP2.3 AT5G47450 拟南芥 Arabidopsis thaliana (L.) Heynh. 液泡膜 NH3转运 [49] -
[1] Zang HD,Blagodatskaya E,Wen Y,Shi LL,Cheng F,et al. Temperature sensitivity of soil organic matter mineralization decreases with long-term n fertilization:evidence from four Q10 estimation approaches[J]. Land Degrad Dev,2020,31 (6):683−693. doi: 10.1002/ldr.3496
[2] Bindraban PS,Dimkpa C,Nagarajan L,Roy A,Rabbinge R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants[J]. Biol Fertil Soils,2015,51 (8):897−911. doi: 10.1007/s00374-015-1039-7
[3] Wang WH,Köhler B,Cao FQ,Liu LH. Molecular and physiological aspects of urea transport in higher plants[J]. Plant Sci,2008,175 (4):467−477. doi: 10.1016/j.plantsci.2008.05.018
[4] Franke W. Mechanisms of foliar penetration of solutions[J]. Annu Rev Plant Physiol,1967,18:281−300. doi: 10.1146/annurev.pp.18.060167.001433
[5] Schreel JDM,Leroux O,Goossens W,Brodersen C,Rubinstein A,Steppe K. Identifying the pathways for foliar water uptake in beech (Fagus sylvatica L. ):a major role for Trichomes[J]. Plant J,2020,103 (2):769−780. doi: 10.1111/tpj.14770
[6] 张俊伶. 植物营养学[M]. 北京: 中国农业大学出版社, 2021: 54-57. [7] Li C,Wang P,van der Ent A,Cheng MM,Jiang HB,et al. Absorption of foliar-applied Zn in sunflower (Helianthus annuus):importance of the cuticle,stomata and Trichomes[J]. Ann Bot,2019,123 (1):57−68. doi: 10.1093/aob/mcy135
[8] Aponte J,Baur P. The role of pH for ionic solute uptake by the non-aerial hypocotyl of mung bean plants[J]. J Plant Dis Prot,2018,125 (4):433−442. doi: 10.1007/s41348-018-0153-9
[9] Fernández V,Bahamonde HA,Peguero-Pina JJ,Gil-Pelegrín E,Sancho-Knapik D,et al. Physico-chemical properties of plant cuticles and their functional and ecological significance[J]. J Exp Bot,2017,68 (19):5293−5306. doi: 10.1093/jxb/erx302
[10] Rosado BHP,Holder CD. The significance of leaf water repellency in Ecohydrological research:a review[J]. Ecohydrology,2013,6 (1):150−161. doi: 10.1002/eco.1340
[11] Goldsmith GR,Bentley LP,Shenkin A,Salinas N,Blonder B,et al. Variation in leaf wettability traits along a tropical montane elevation gradient[J]. New Phytol,2017,214 (3):989−1001. doi: 10.1111/nph.14121
[12] Eichert T,Goldbach HE. Equivalent pore radii of hydrophilic foliar uptake routes in Stomatous and Astomatous leaf surfaces-further evidence for a stomatal pathway[J]. Physiol Plant,2008,132 (4):491−502. doi: 10.1111/j.1399-3054.2007.01023.x
[13] Yamada Y,Wittwer SH,Bukovac MJ. Penetration of organic compounds through isolated cuticular membranes with special reference to C14 Urea[J]. Plant Physiol,1965,40 (1):170−175. doi: 10.1104/pp.40.1.170
[14] Johnson HB. Plant pubescence:an ecological perspective[J]. Bot Rev,1975,41 (3):233−258. doi: 10.1007/BF02860838
[15] 程丽林, 谷海萍, 刘梦琳, 张长峰. 植物果实角质层及其渗透性的研究进展[J]. 保鲜与加工, 2019, 19(3): 174-178. Cheng LL, Gu HP, Liu ML, Zhang CF. Research progress on the plantfruit cuticles and its permeability[J]. Storage and Process, 2019, 19(3): 174-178.
[16] Waseem M,Nie ZF,Yao GQ,Hasan M,Xiang Y,Fang XW. Dew absorption by leaf Trichomes in Caragana korshinskii:an alternative water acquisition strategy for withstanding drought in arid environments[J]. Physiol Plant,2021,172 (2):528−539. doi: 10.1111/ppl.13334
[17] Schreel JDM,Steppe K. Foliar water uptake in trees:negligible or necessary?[J]. Trends Plant Sci,2020,25 (6):590−603. doi: 10.1016/j.tplants.2020.01.003
[18] Yan A,Pan JB,An LZ,Gan YB,Feng HY. The responses of Trichome mutants to enhanced ultraviolet-B radiation in Arabidopsis thaliana[J]. J Photochem Photobiol B:Biol,2012,113:29−35. doi: 10.1016/j.jphotobiol.2012.04.011
[19] Villar-Salvador P,Planelles R,Oliet J,Peñuelas-Rubira JL,Jacobs DF,González M. Drought tolerance and transplanting performance of holm oak (Quercus ilex) seedlings after drought hardening in the nursery[J]. Tree Physiol,2004,24 (10):1147−1155. doi: 10.1093/treephys/24.10.1147
[20] Bethea Jr FG,Park D,Mount A,Menchyk N,Liu HB. Effects of acute moisture stress on creeping bentgrass cuticle morphology and associated effects on foliar nitrogen uptake[J]. HortScience,2014,49 (12):1582−1587. doi: 10.21273/HORTSCI.49.12.1582
[21] 刘海光,罗振,董合忠. 植物硝态氮吸收和转运的调控研究进展[J]. 生物技术通报,2021,37(6):192−201. Liu HG,Luo Z,Dong HZ. Research progress on the regulation of NO3- uptake and transport in plant[J]. Biotechnology Bulletin,2021,37 (6):192−201.
[22] Liu LH,Ludewig U,Gassert B,Frommer WB,Von Wirén N. Urea transport by nitrogen-regulated tonoplast intrinsic proteins in Arabidopsis[J]. Plant Physiol,2003,133 (3):1220−1228. doi: 10.1104/pp.103.027409
[23] Kojima S,Bohner A,Gassert B,Yuan LX,Wirén NV. AtDUR3 represents the major transporter for high-affinity urea transport across the plasma membrane of nitrogen-deficient Arabidopsis roots[J]. Plant J,2007,52 (1):30−40. doi: 10.1111/j.1365-313X.2007.03223.x
[24] Liu LH,Ludewig U,Frommer WB,Von Wirén N. AtDUR3 encodes a new type of high-affinity urea/H + symporter in Arabidopsis[J]. Plant Cell,2003,15 (3):790−800. doi: 10.1105/tpc.007120
[25] Liu GW,Sun AL,Li DQ,Athman A,Gilliham M,Liu LH. Molecular identification and functional analysis of a maize (Zea mays) DUR3 homolog that transports urea with high affinity[J]. Planta,2015,241 (4):861−874. doi: 10.1007/s00425-014-2219-7
[26] Beier MP,Fujita T,Sasaki K,Kanno K,Ohashi M,et al. The urea transporter DUR3 contributes to rice production under nitrogen-deficient and field conditions[J]. Physiol Plant,2019,167 (1):75−89. doi: 10.1111/ppl.12872
[27] Wang WH,Köhler B,Cao FQ,Liu GW,Gong YY,et al. Rice DUR3 mediates high-affinity urea transport and plays an effective role in improvement of urea acquisition and utilization when expressed in Arabidopsis[J]. New Phytol,2012,193 (2):432−444. doi: 10.1111/j.1469-8137.2011.03929.x
[28] Zanin L,Tomasi N,Wirdnam C,Meier S,Komarova NY,et al. Isolation and functional characterization of a high affinity urea transporter from roots of Zea mays[J]. BMC Plant Biol,2014,14 (1):222. doi: 10.1186/s12870-014-0222-6
[29] Kakinuma M,Coury DA,Nakamoto C,Sakaguchi K,Amano H. Molecular analysis of physiological responses to changes in nitrogen in a marine Macroalga,Porphyra yezoensis(Rhodophyta)[J]. Cell Biol Toxicol,2008,24 (6):629−639. doi: 10.1007/s10565-007-9053-7
[30] Kakinuma M,Suzuki K,Iwata S,Coury DA,Iwade S,Mikami K. Isolation and characterization of a new DUR3-like gene,PyDUR3.3,from the marine macroalga Pyropia yezoensis (Rhodophyta)[J]. Fish Sci,2016,82 (1):171−184. doi: 10.1007/s12562-015-0947-7
[31] Gaspar M,Bousser A,Sissoëff I,Roche O,Hoarau J,Mahé A. Cloning and characterization of ZmPIP1-5b,an aquaporin transporting water and urea[J]. Plant Sci,2003,165 (1):21−31. doi: 10.1016/S0168-9452(03)00117-1
[32] Gu RL,Chen XL,Zhou YL,Yuan LX. Isolation and characterization of three maize aquaporin genes,ZmNIP2;1,ZmNIP2;4 and ZmTIP4;4 involved in urea transport[J]. BMB Rep,2012,45 (2):96−101. doi: 10.5483/BMBRep.2012.45.2.96
[33] Mitani N,Yamaji N,Ma JF. Characterization of substrate specificity of a rice silicon transporter,Lsi1[J]. Pflügers Archiv Eur J Physiol,2008,456 (4):679−686.
[34] Wallace IS,Roberts DM. Distinct transport selectivity of two structural subclasses of the Nodulin-like intrinsic protein family of plant Aquaglyceroporin channels[J]. Biochemistry,2005,44 (51):16826−16834. doi: 10.1021/bi0511888
[35] Yang H,Menz J,Häussermann I,Benz M,Fujiwara T,Ludewig U. High and low affinity urea root uptake:involvement of NIP5;1[J]. Plant Cell Physiol,2015,56 (8):1588−1597. doi: 10.1093/pcp/pcv067
[36] 石永春,郭尧,薛瑞丽. 烟草硼内向转运体NIP5;1的克隆和表达分析[J]. 中国农学通报.,2016,32(21):100−105. Shi YC,Guo Y,Xue RL. Cloning and expression analysis of boron infux transporter NIP5;1 form Nicotiana[J]. Chinses Agricultual Science Bulletin,2016,32 (21):100−105.
[37] Klebl F,Wolf M,Sauer N. A defect in the yeast plasma membrane urea transporter Dur3p is complemented by CpNIP1,a Nod26-like protein from zucchini (Cucurbita pepo L.),and by Arabidopsis thaliana δ-TIP or γ-TIP[J]. FEBS Lett,2003,547 (1-3):69−74. doi: 10.1016/S0014-5793(03)00671-9
[38] Soto G,Fox R,Ayub N,Alleva K,Guaimas F,et al. TIP5;1 is an aquaporin specifically targeted to pollen mitochondria and is probably involved in nitrogen remobilization in Arabidopsis thaliana[J]. Plant J,2010,64 (6):1038−1047. doi: 10.1111/j.1365-313X.2010.04395.x
[39] Gerbeau P,Güçlü J,Ripoche P,Maurel C. Aquaporin Nt-TIPa can account for the high permeability of tobacco cell vacuolar membrane to small neutral solutes[J]. Plant J,1999,18 (6):577−587. doi: 10.1046/j.1365-313x.1999.00481.x
[40] Matiz A,Cambuí CA,Richet N,Mioto PT,Gomes F,et al. Involvement of Aquaporins on nitrogen-acquisition strategies of juvenile and adult plants of an epiphytic tank-forming bromeliad[J]. Planta,2019,250 (1):319−332. doi: 10.1007/s00425-019-03174-7
[41] Bienert GP,Bienert MD,Jahn TP,Boutry M,Chaumont F. Solanaceae XIPs are plasma membrane Aquaporins that facilitate the transport of many uncharged substrates[J]. Plant J,2011,66 (2):306−317. doi: 10.1111/j.1365-313X.2011.04496.x
[42] Wang M,Ding L,Gao LM,Li YR,Shen QR,Guo SW. The interactions of Aquaporins and mineral nutrients in higher plants[J]. Int J Mol Sci,2016,17 (8):1229. doi: 10.3390/ijms17081229
[43] Miwa K, Tanaka M, Kamiya T, Fujiwara T. Molecular mechanisms of boron transport in plants: involvement of Arabidopsis NIP5;1 and NIP6;1[M]//Jahn TP, Bienert GP, eds. MIPs and Their Roles in the Exchange of Metalloids. New York: Springer, 2010: 83-96.
[44] Zhao FJ,Ago Y,Mitani N,Li RY,Su YH,et al. The role of the rice aquaporin Lsi1 in Arsenite efflux from roots[J]. New Phytol,2010,186 (2):392−399. doi: 10.1111/j.1469-8137.2010.03192.x
[45] Maeshima M. Tonoplast transporters:organization and function[J]. Annu Rev Plant Physiol Plant Mol Biol,2001,52:469−497. doi: 10.1146/annurev.arplant.52.1.469
[46] Jahn TP,Møller ALB,Zeuthen T,Holm LM,Klaerke DA,et al. Aquaporin homologues in plants and mammals transport ammonia[J]. FEBS Lett,2004,574 (1-3):31−36.
[47] Quiroga G,Erice G,Aroca R,Delgado-Huertas A,Ruiz-Lozano JM. Elucidating the possible involvement of maize Aquaporins and arbuscular mycorrhizal symbiosis in the plant ammonium and urea transport under drought stress conditions[J]. Plants,2020,9 (2):148. doi: 10.3390/plants9020148
[48] Chrispeels MJ,Maurel C. Aquaporins:the molecular basis of facilitated water movement through living plant cells?[J]. Plant Physiol,1994,105 (1):9−13. doi: 10.1104/pp.105.1.9
[49] Loque D,Ludewig U,Yuan LX,von Wirén N. Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole[J]. Plant Physiol,2005,137 (2):671−680. doi: 10.1104/pp.104.051268
[50] Gupta AB,Sankararamakrishnan R. Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa:characterization of XIP subfamily of Aquaporins from evolutionary perspective[J]. BMC Plant Biol,2009,9:134. doi: 10.1186/1471-2229-9-134
[51] Witte CP,Tiller SA,Taylor MA,Davies HV. Leaf urea metabolism in potato. Urease activity profile and patterns of recovery and distribution of 15N after foliar urea application in wild-type and urease-antisense Transgenics[J]. Plant Physiol,2002,128 (3):1129−1136. doi: 10.1104/pp.010506
[52] 张海涛,宋远辉,郑思怡,花芹,杨晔,林泉祥. 植物尿素代谢及稳态调节机制研究进展[J]. 安徽农业大学学报,2022,49(2):181−188. Zhang HT,Song YH,Zheng SY,Hua Q,Yang Y,Lin QX. Research progress on urea metabolism and regulation mechanism of homeostasis in plants[J]. Journal of Anhui Agricultural University,2022,49 (2):181−188.
[53] 曹凤秋,刘国伟,王伟红,吴学明,刘来华. 高等植物尿素代谢及转运的分子机理[J]. 植物学报,2009,44(3):273−282. Cao FJ,Liu GW,Wang WH,Wu XM,Liu LH. Molecular processes of urea metabolism and transport in higher plants[J]. Chinese Bulletin of Botany,2009,44 (3):273−282.
[54] Machuca A,Cuba-Díaz M,Córdova C. Enzymes in the rhizosphere of plants growing in the vicinity of the polish Arctowski Antarctic station[J]. J Soil Sci Plant Nutr,2015,15 (4):833−838.
[55] De Macedo FG,Bresolin JD,Santos EF,Furlan F,Lopes da Silva WT,et al. Nickel availability in soil as influenced by liming and its role in soybean nitrogen metabolism[J]. Front Plant Sci,2016,7:1358.
[56] Kanamori T,Kanou N,Atomi H,Imanaka T. Enzymatic characterization of a prokaryotic urea carboxylase[J]. J Bacteriol,2004,186 (9):2532−2539.
[57] Tegeder M,Masclaux-Daubresse CE. Source and sink mechanisms of nitrogen transport and use[J]. New Phytologist,2017,217 (1):35−53.
[58] 王慧飞,冯雪,张一名,陈光,孙艳香. 植物中尿素循环相关酶及代谢产物研究进展[J]. 云南农业大学学报(自然科学),2018,33(2):334−342. Wang HF,Feng X,Zhang YM,Chen G,Sun YX. Advance in study on the urea cycle related enzymes and metabolic products in plant[J]. Journal of Yunnan Agricultural University (Natural Science)
,2018,33 (2):334−342. [59] Ariz I,Asensio AC,Zamarreño AM,García-Mina JM,Aparicio-Tejo PM,Moran JF. Changes in the C/N balance caused by increasing external ammonium concentrations are driven by carbon and energy availabilities during ammonium nutrition in pea plants:the key roles of asparagine synthetase and anaplerotic enzymes[J]. Physiol Plant,2013,148 (4):522−537. doi: 10.1111/j.1399-3054.2012.01712.x
[60] Todd CD,Tipton PA,Blevins DG,Piedras P,Pineda M,Polacco JC. Update on Ureide degradation in legumes[J]. J Exp Bot,2006,57 (1):5−12. doi: 10.1093/jxb/erj013
[61] 张文婧,林琳,孙威江. 茶树铵转运蛋白AMT研究进展[J]. 分子植物育种,2022,20(8):2586−2596. Zhang WJ,Lin L,Sun WJ. Advances of ammonium transporter AMT in tea plant (Camellia sinensis (L.) O. Ktze.)[J]. Molecular Plant Breeding,2022,20 (8):2586−2596.
[62] Kobae Y,Tamura Y,Takai S,Banba M,Hata S. Localized expression of arbuscular mycorrhiza-inducible ammonium transporters in soybean[J]. Plant Cell Physiol,2010,51 (9):1411−1415. doi: 10.1093/pcp/pcq099
[63] Pearson JN,Finnemann J,Schjoerring JK. Regulation of the high-affinity ammonium transporter (BnAMT1;2) in the leaves of Brassica napus by nitrogen status[J]. Plant Mol Biol,2002,49 (5):483−490. doi: 10.1023/A:1015549115471
[64] Couturier J,Montanini B,Martin F,Brun A,Blaudez D,Chalot M. The expanded family of ammonium transporters in the perennial poplar plant[J]. New Phytol,2007,174 (1):137−150. doi: 10.1111/j.1469-8137.2007.01992.x
[65] Von Wiren N,Lauter FR,Ninnemann O,Gillissen B,Walch-Liu P,et al. Differential regulation of three functional ammonium transporter genes by nitrogen in root hairs and by light in leaves of tomato[J]. Plant J,2000,21 (2):167−175. doi: 10.1046/j.1365-313x.2000.00665.x
[66] Bindel N,Neuhäuser B. High-affinity ammonium transport by Arabidopsis thaliana AMT1;4[J]. Acta Physiol Plant,2021,43 (4):69. doi: 10.1007/s11738-021-03235-z
[67] Sohlenkamp C,Wood CC,Roeb GW,Udvardi MK. Characterization of Arabidopsis AtAMT2,a high-affinity ammonium transporter of the plasma membrane[J]. Plant Physiol,2002,130 (4):1788−1796. doi: 10.1104/pp.008599
-
期刊类型引用(1)
1. 惠生娟,葛丽萍,王子瑜,张玉胜,苏云婷,孙岩,李润植. 续随子MYB基因家族的鉴定及ElMYB114在油脂合成中的功能分析. 植物科学学报. 2025(01): 92-101 .  本站查看
本站查看
其他类型引用(0)



 下载:
下载: