Effects of soil nitrogen addition on photosynthetic limitations in Fraxinus mandshurica Rupr. and Quercus mongolica Fish. ex Ledeb
-
摘要:
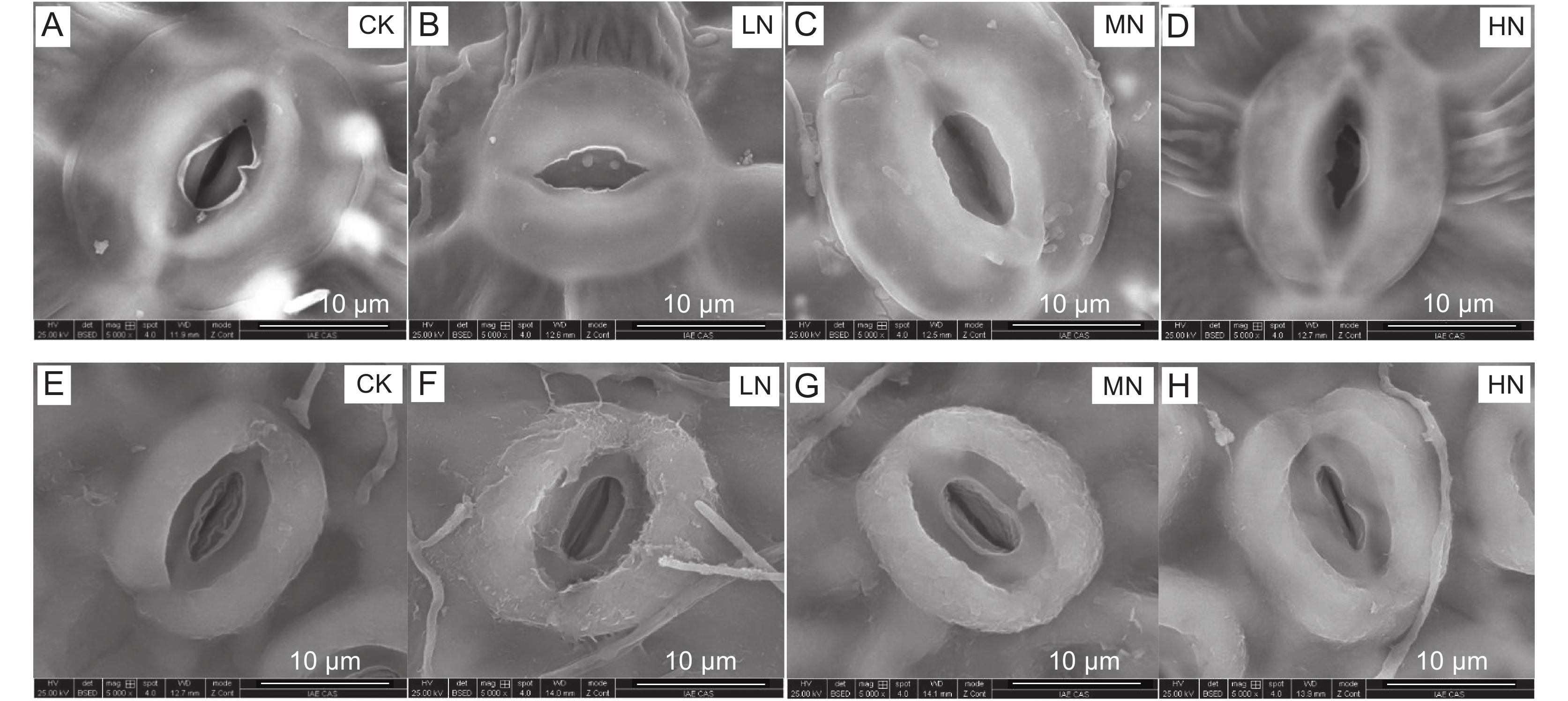
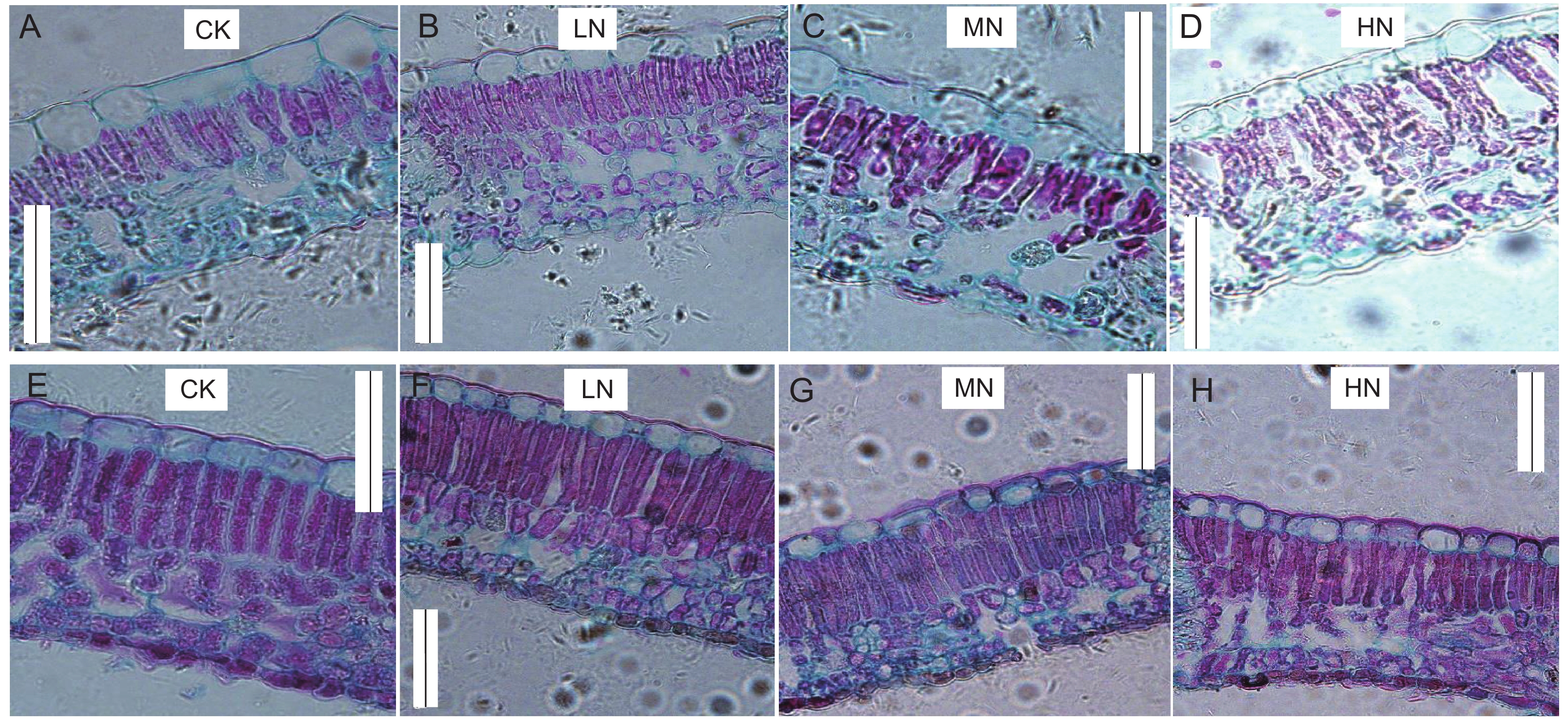
本文以试验地常年大气氮(N)沉降量(23 kg·ha−1·year−1)为依据,设计了低度(LN,23 kg·ha−1·year−1)、中度(MN,46 kg·ha−1·year−1)和高度(HN,69 kg·ha−1·year−1)3种氮添加水平以模拟大气氮沉降,以无氮添加处理为对照(CK),探究过度氮沉降对森林阔叶树种水曲柳(Fraxinus mandshurica Rupr.)和蒙古栎(Quercus mongolica Fish. ex Ledeb)的生理生态效应。结果显示:(1)两树种CO2扩散性限制作用(即气孔限制lsc和叶肉限制lm)在氮添加后减弱,而后随氮量的增加先减弱后增强;其生化限制lb则在氮添加后增强,后随氮量增加先增强后减弱;(2)3种光合限制作用均在MN下达到最值, 中度土壤氮添加量对植株光合的促进效应最高;(3)土壤氮添加期间植株光合能力的增强主要源于CO2扩散性限制作用的减弱,而gsc变化(即lsc)为扩散性限制的主角作用因子;(4)3种光合限制作用(lsc、lm和lb)在不同生长期(7月和8月)均未表现出显著差异,表明lsc的光合主角限制“角色”无季节性差异;(5)一定范围内的土壤氮添加不会对植株的水分利用潜力产生显著影响。
Abstract:Based on natural nitrogen deposition in the field (23 kg·ha−1·year−1), this research employed low (LN, 23 kg·ha−1·year−1), moderate (MN, 46 kg·ha−1·year−1), and high (HN, 69 kg·ha−1·year−1) nitrogen levels to simulate natural nitrogen deposition, using no nitrogen addition used as a control (CK). The goal was to explore the physiological and ecological effects of excessive nitrogen deposition on two broad-leaved forest species, i.e., Manchurian ash (Fraxinus mandshurica Rupr.) and Mongolian oak (Quercus mongolica Fish. ex Ledeb). Results showed that 1) CO2 diffusional limitations (i.e., stomatal limitation, lsc, mesophyll limitation, lm) of both species decreased by more than 10% after nitrogen addition, then increased with increasing nitrogen supply, while biochemical limitation (lb) increased by more than 10% after nitrogen addition, then decreased with increasing nitrogen supply. 2) Both lsc and lm reached minimum values of 18.4% and 18.0% (Manchurian ash-August), 21.6% and 19.7% (Mongolian oak-July), and 21.6% and 20.1% (Mongolian oak-August), while lb reached a maximum value of 63.6% (Manchurian ash-August) and 59.7% and 58.3% (Mongolian oak-July and August) under MN treatment, indicating that soil nitrogen addition of 46 kg·ha-1·year-1 had the greatest photosynthesis-promoting effect. 3) The enhancement of plant photosynthetic capacity during soil nitrogen supply predominantly resulted from the weakening of CO2 diffusional limitations, in which stomatal conductance to CO2 (gsc, i.e., lsc) was the primary limiting factor affecting plant photosynthesis. 4) The three photosynthetic limitations (lsc, lm, and lb) did not show any significant differences between July and August, indicating that the primary photosynthetic role of lsc may lack seasonal variation. 5) Soil nitrogen addition within a certain content range did not significantly affect the water use potential of plants.
-
能源安全是世界各国经济可持续发展的保障。鉴于传统化石能源日趋短缺和过度消耗造成的环境污染等,开发可再生绿色能源已成为全球关注的议题。生物质是可再生、可持续的生物能源重要原材料。续随子(Euphorbia lathyris L.)作为一种能源油料植物,具有较高的开发潜力和价值。续随子是大戟科大戟属的草本植物,在我国分布范围广泛,于江苏、浙江、福建、湖南、湖北、广西、四川等10余个省均有分布[1]。生长期一般为1~2年,且具有耐旱、喜温暖湿润、喜光照、怕水涝等特点[2]。种子油类似于石油的碳氢化合物,经过适当加工处理可制备生物燃油,具有良好的燃烧性能[3]。续随子种子油含量高达45%~60%,脂肪酸成分包括棕榈酸(16∶0)、硬脂酸(18∶0)、油酸(18∶1)、亚油酸(18∶2)、亚麻酸(18∶3)等,其中油酸含量高达总油脂含量的85%以上,棕榈酸和亚油酸分别约为8%,硬脂酸和亚麻酸分别约为2.5%[4, 5]。续随子是所有经过检测的油料植物中油酸含量最高的作物[6]。
WRI1是一种AP2/EREBP型转录因子,也是调控植物油脂合成的关键因子。这类转录因子均具有两个典型的AP2保守结构域[7],其中一个相对保守,大约60个氨基酸,另一个结构域保守性较差,且氨基酸数目较多。AP2保守结构域的末端有许多Ser和Thr氨基酸残基,C端有一个酸性结构域。如果去除C端的酸性结构域,转录活性将被阻止[8]。目前已经在多种植物中发现WRI1与体内油脂生物合成相关,主要是通过激活糖酵解及脂肪酸合成过程中的相关基因,从而促进油脂的合成[9]。WRI1可激活许多参与质体中脂肪酸从头合成的基因,包括编码质体型丙酮酸激酶(Pl-PKb1)、乙酰辅酶a羧化酶(BCCP2)、酰基载体蛋白(ACP1)和酮酰酰基载体蛋白合成酶(KAS1)的基因[10]。
在玉米(Zea mays L.)中过表达WRI1基因,可使种子含油量提高48%,且植物生长和产量并未受影响[11]。在拟南芥(Arabidopsis thaliana (L.) Heynh.)中异源表达油菜(Brassica napus L.)WRI1基因,种子中油脂含量提高了10%~40%[12]。在本氏烟草(Nicotiana benthamiana L.)中瞬时表达向日葵(Helianthus annuus L.)HaWRI1可促进其叶片油脂的大量积累[13]。在油菜和大豆(Glycine max (L.) Merr.)[14]等植物中也克隆出了WRI1基因。然而,有关续随子WRI1基因的研究还未见报道。
本研究基于全基因组序列,鉴定续随子的WRI1蛋白,对续随子ElWRI1基因的表达模式进行分析,进一步构建表达载体,并在本氏烟草叶片中进行瞬时异源表达,检测转基因叶片组织的总油脂含量及脂肪酸成分变化。研究结果旨在为全面解析续随子ElWRI1调控油脂生物合成的分子机制提供参考,并为其他植物油脂代谢遗传修饰途径研究提供新思路。
1. 材料与方法
1.1 材料
本研究所用的续随子种质材料来自山西农业大学林学院苗圃。实验采集续随子的根、茎、叶、花以及不同发育时期(分别为花后15(S1)、30(S2)、45 d(S3))的种子作为研究材料。
1.2 方法
1.2.1 ElWRI1蛋白的生物信息学分析
以从拟南芥数据库(TAIR-Home Page)获得的AtWRI1蛋白(AT3G54320)序列为模板,对续随子基因组(https://doi.org/10.6084/m9.figshare.14909913.v1.)进行BLASTP分析,鉴定获得11种长度不同的续随子ElWRI1蛋白。
使用ProParam(http://expasy.org/tools/protparam.html)软件预测ElWRI1蛋白的理论等电点、不稳定系数和疏水性等理化性质。运用MEGA 7.0软件对续随子WRI1蛋白和其他物种已知功能的WRI1蛋白序列构建系统进化树。用ProtFun(http://www.cbs.dtu.dk/services/ ProtFun/)在线软件预测蛋白质的功能分类,用SMART(http://smart.embl-heidelberg.de/)在线工具对续随子WRI1蛋白的功能结构域进行分析。利用PRALINE(http://www.ibi.vu.nl/programs/pralinewww/)在线工具对续随子和拟南芥的WRI1蛋白进行氨基酸序列多重比对分析。
1.2.2 种子不同发育时期续随子ElWRI1的表达分析
依据续随子种子发育进程,划分为3个时期(分别为花后15、30及45 d)进行取样,提取总RNA,构建cDNA文库,测序获得续随子发育种子的转录组数据(未发表数据)。依据注释的ElWRI1序列,计算各个ElWRI1成员的表达量(FPKM),绘制表达热图。
1.2.3 ElWRI1-8的基因克隆
使用TRNzol试剂盒(天根,北京)提取续随子根、茎、叶、花和3个不同发育时期种子的总RNA,反转录合成cDNA,以此为模板,特异性扩增ElWRI1-8的ORF序列。ElWRI1-8的全长 cDNA 用特异性引物扩增(ElWRI1-8-F:5'-GCTCCTCTTCATCCTCTTCTTC -3';ElWRI1-8-R:5'-ATGCCTGGTAACTCCTCTGTAA-3')。随后,使用 EasyPfu DNA 聚合酶通过RT-PCR将 cDNA用于扩增 ORF。将扩增子分别克隆到pGEM T-easy载体中。将阳性菌株送往安徽通用生物测序,测序结果与序列完全匹配。
1.2.4 ElWRI1-8表达载体的构建及瞬时表达
使用具有 Xba Ⅰ(5'-末端)和 Kpn Ⅰ(3'-末端)位点的基因特异性引物(F:5'-GCTCTAGAATGGGAAACAAGGAAACAATGG -3'; R:5'-CCCCCGGGCTACAACACTCTCAGTTGAAGAT-3'),从相应的克隆载体中分别扩增ElWRI1-8。然后将其插入 pCAMBIA1303载体中。再将含有ElWRI1-8的载体通过冻融方法转化到根癌农杆菌GV3101中。参考霍琳等[15]的方法侵染烟草叶片并取样。
1.2.5 脂肪酸甲酯和总油脂测定
参考任国鹏等[16]的方法提取转基因烟草叶片的总油脂和脂肪酸。参考王计平等[17]及李璐[18]的方法,使用安捷伦7890B气象色谱仪(Agilent,美国)测定烟草叶片脂肪酸各组分含量,脂肪酸含量利用安捷伦软件进行分析。
2. 结果与分析
2.1 ElWRI1的全基因组鉴定及蛋白保守基序分析
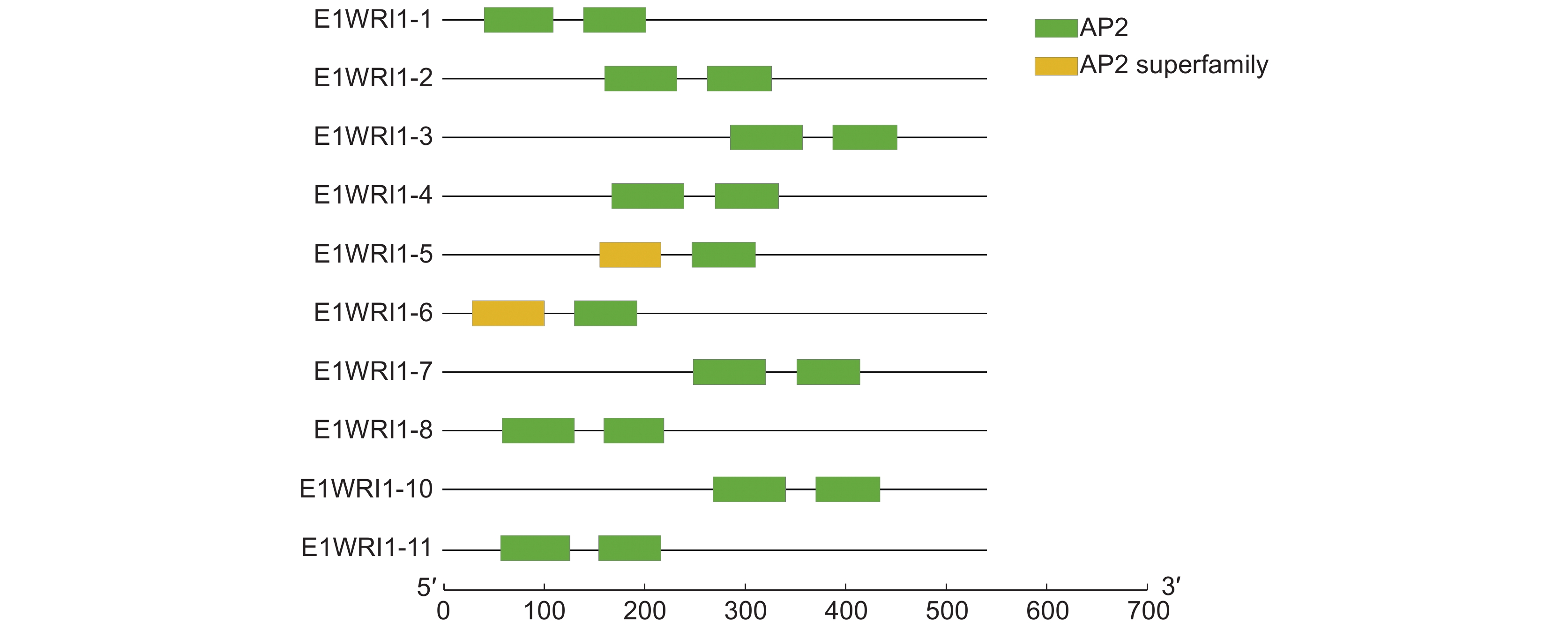
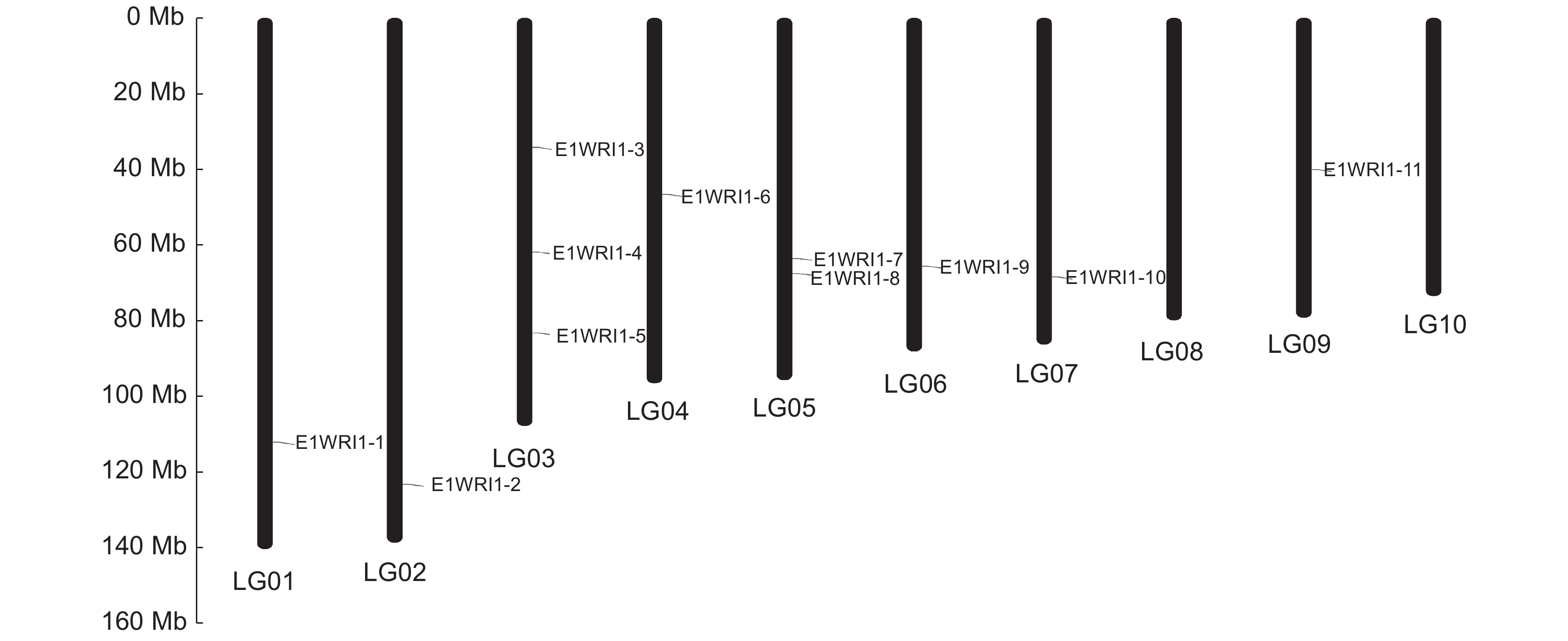
应用 NCBI 网站的Genome Date Viewer工具,获得续随子WRI1各基因的染色体信息(图1)。续随子WRI1基因分布于 1~7和9号染色体上,分别命名为 WRI1-1~WRI1-11。
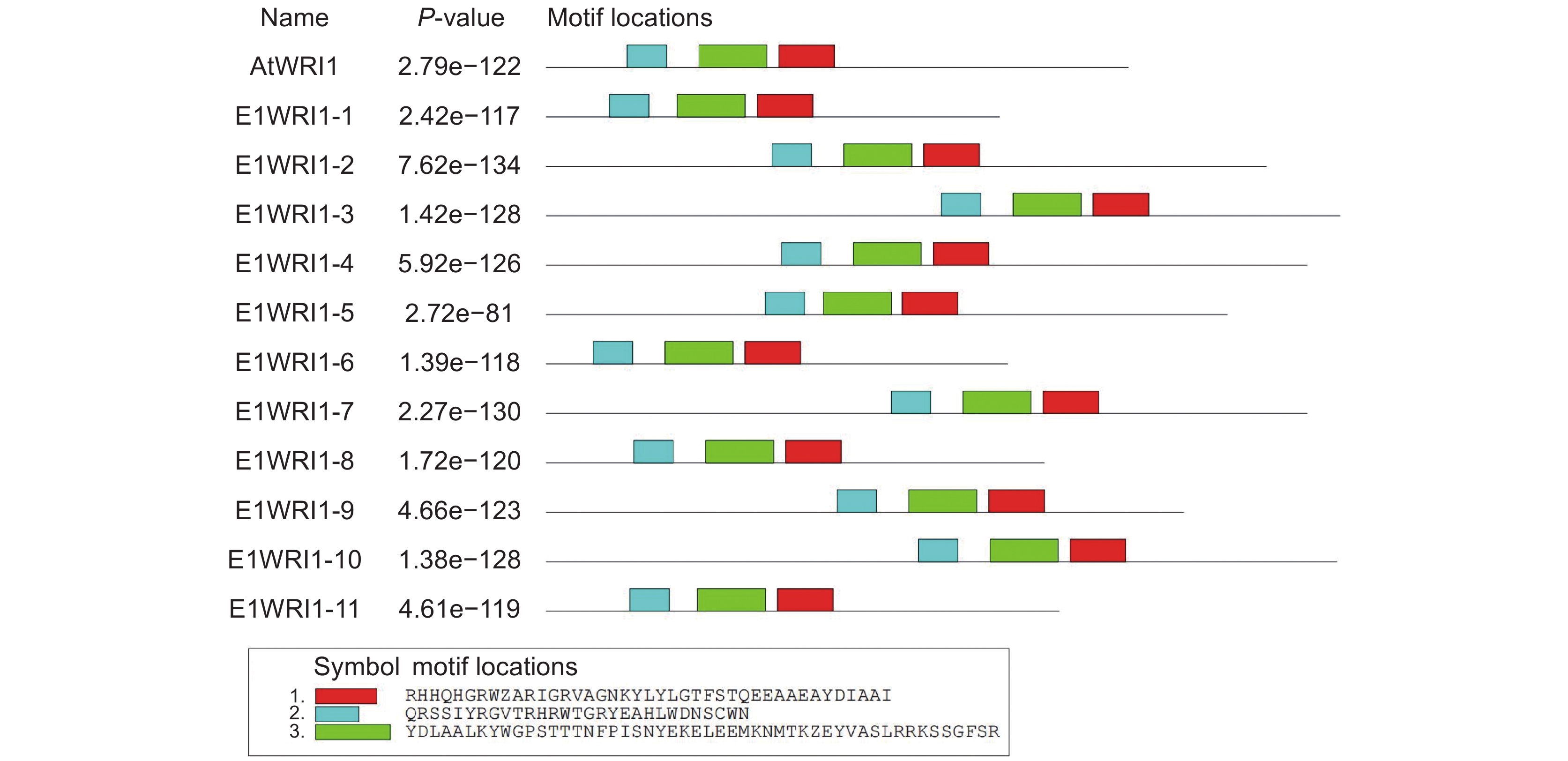
通过CDD鉴定WRI1的功能结构域,结果如图2所示。11个WRI1蛋白均含有两个AP2保守结构域,均属于AP2/EREBP家族。续随子ElWRI1蛋白保守基序分析结果如图3所示,ElWRI1蛋白具有相似的基序排列,均包含3个motif。这也表明这些蛋白质具有类似的功能。
2.2 续随子WRI1基因结构分析
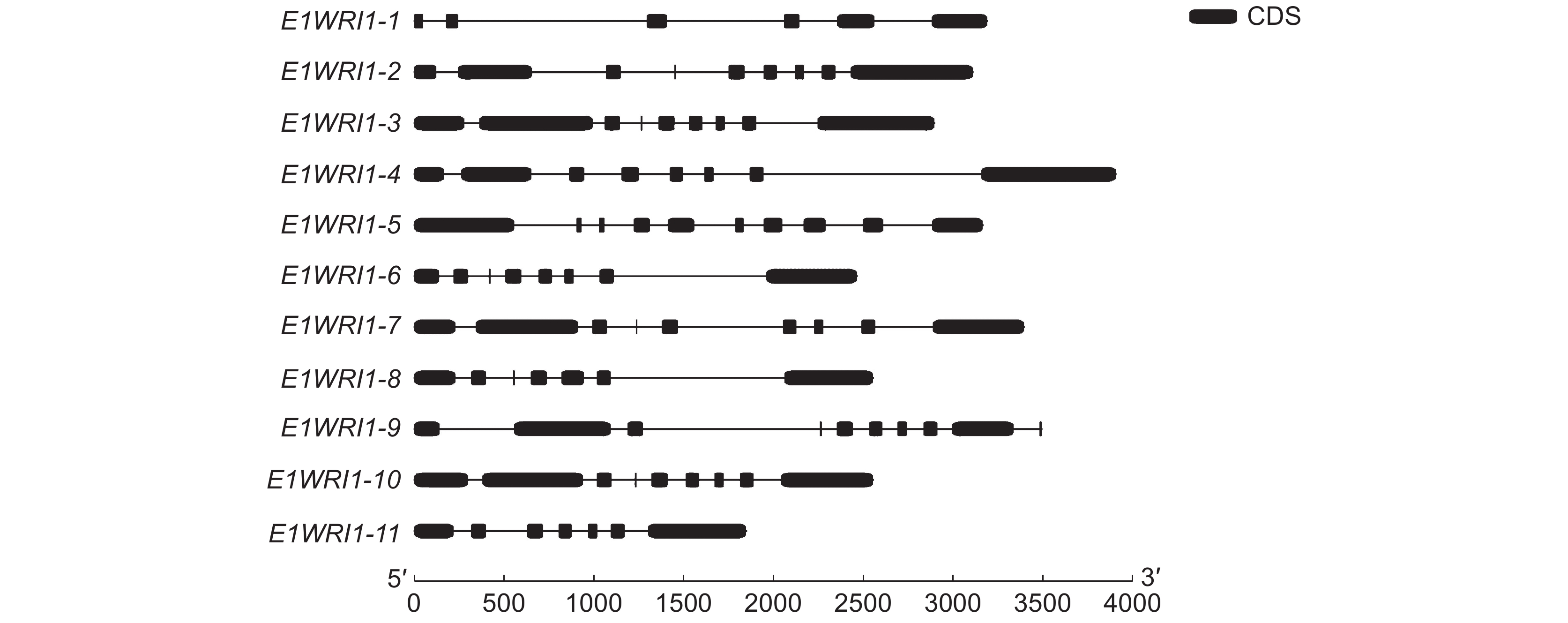
利用 GSDS 2.0网站分析WRI1的基因结构,结果显示(图4),WRI1-4序列最长,WRI1-11序列最短。此外,WRI1-6、WRI1-8、WRI1-11基因结构中包含7个外显子和6个内含子,而WRI1-2、WRI1-3、WRI1-7和WRI1-9基因均有9个外显子和8个内含子,WRI1-4和WRI1-10都有8个外显子和7个内含子。内含子和外显子数量最多的是WRI1-5,有10个外显子和9个内含子。内含子和外显子数量最少的是WRI1-1,有5个外显子和4个内含子。
2.3 续随子WRI1蛋白的理化性质分析
续随子WRI1蛋白的基本理化性质见附表1
1 ,WRI1蛋白含334 ~ 646个氨基酸,相对分子量为 38.25 ~ 71.97 kD,其中WRI1-3氨基酸最多,相对分子量最大,WRI1-1的氨基酸最少,相对分子量最小。WRI1-1、WRI1-6、WRI1-8和WRI1-11的理论等电点大于7,为碱性蛋白,其余蛋白的等电点均小于7,为酸性蛋白。11个ElWRI1转录因子均为不稳定蛋白。亲水性指数均为负值,表明ElWRI1蛋白为亲水性蛋白。所有WRI1蛋白均无跨膜结构,且预测均定位于细胞核。此外,WRI1-3、WRI1-6、WRI1-8、WRI1-10和WRI1-11存在信号肽序列,为分泌蛋白。2.4 续随子ElWRI1蛋白的高级结构预测
续随子ElWRI1蛋白二级结构预测结果见附表2
1 。WRI1蛋白的二级结构由无规则卷曲、α螺旋、延伸链和β-折叠组成。每个WRI1蛋白二级结构的主体相似,但各个组成结构的比例存在一定差异,均为α螺旋和无规则卷曲所占比例最高,其中β折叠所占比例最低。2.5 续随子ElWRI1蛋白多序列比对和系统进化分析
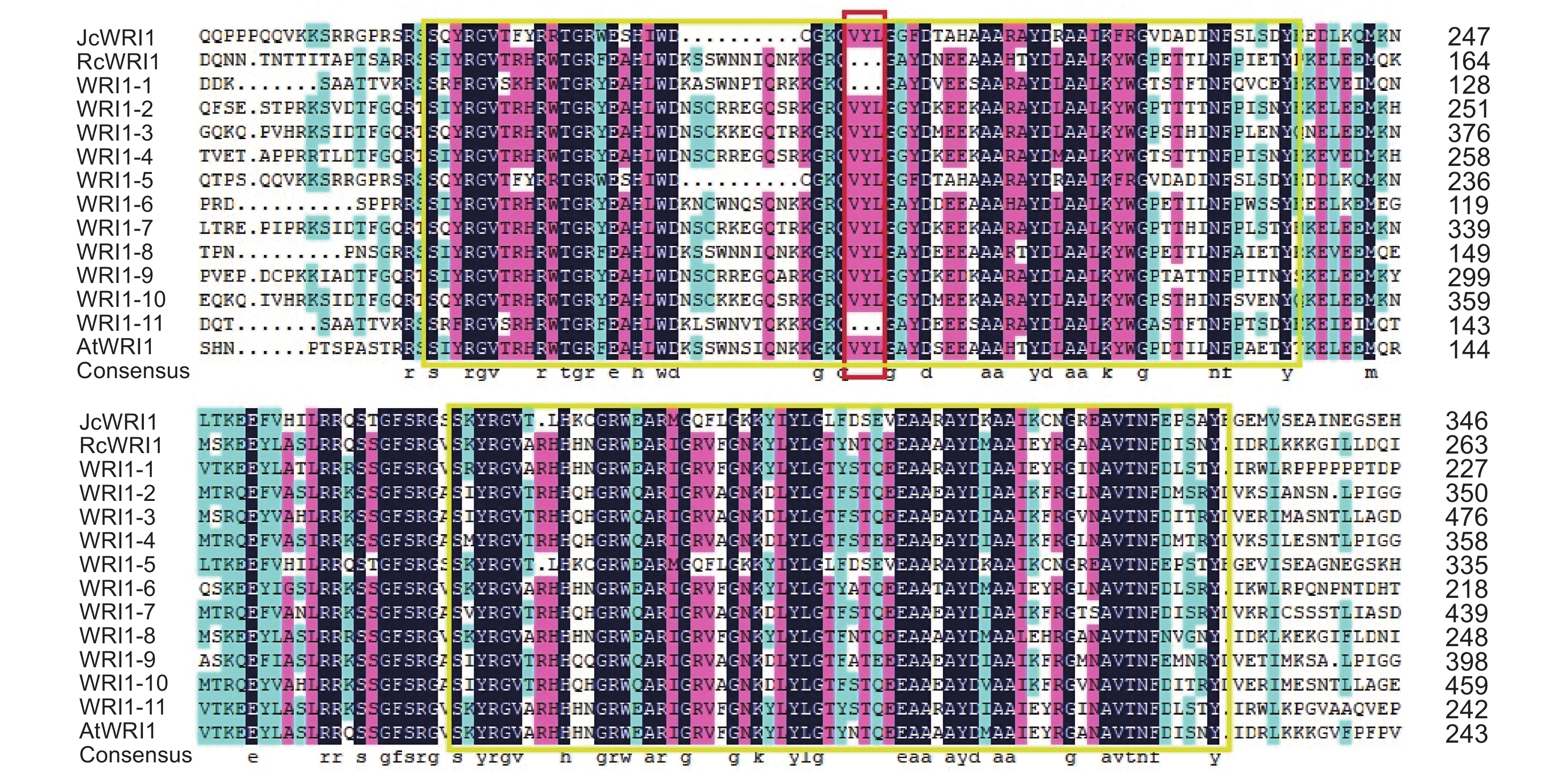
选择同属于大戟科的蓖麻(Ricinus communis L.)RcWRI1和麻风树(Jatropha curcas L.)JcWRI1以及模式植物拟南芥AtWRI1为参考序列,利用DNAman软件对续随子ElWRI1蛋白进行多序列比对分析,结果如图5所示。续随子WRI1与麻风树JcWRI1、蓖麻RcWRI1和拟南芥AtWRI1相似,均包含2个相对保守的AP2/EREBP结构域区域。除ElWRI1-1和ElWRI1-11之外,其余ElWRI1蛋白序列均具有“VYL”氨基酸结构。
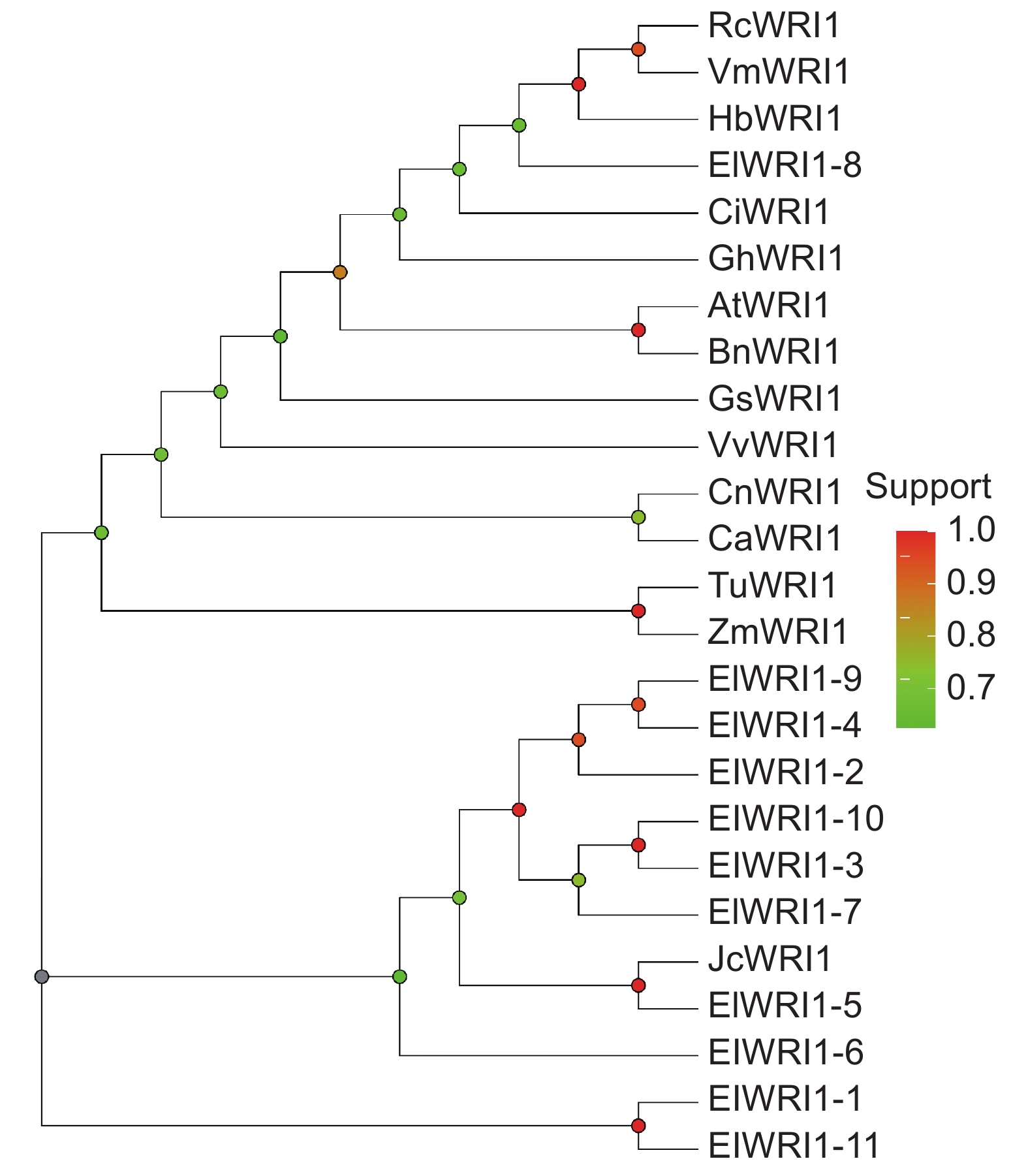
系统发育分析结果显示(图6),ElWRI1-2、ElWRI1-3、ElWRI1-4、ElWRI1-9、ElWRI1-7和ElWRI1-10的亲缘关系较近,聚为一支;ElWRI1-5与麻风树JcWRI1聚为一支;ElWRI1-8则与蓖麻RcWRI1、巴西橡胶树(Hevea brasiliensis(Willd. ex A. Juss.)Muell. Arg.)HbWRI1、皱果桐(Vernicia montana Lour.)VmWRI1聚在一起。
![]() 图 6 不同物种WRI1蛋白的系统发育分析RcWRI1:蓖麻 (NP_001310691.1);AtWRI1:拟南芥 (OAP01705.1);JcWRI1:麻风树 (AIR74897.1);HbWRI1:巴西橡胶树 (XP_021674385.1);ZmWRI1:玉米 (ONM19989.1);BnWRI1:甘蓝型油菜 (ADO16346.1);CsWRI1:大豆 (KHN10130.1);VvWRI1:葡萄 (XP_010659009.1); CaWARI:辣椒(KAF3665339.1); CiWRI1:山核桃 (QQP23243.1); CnWRI1: 椰子 (KAG1365549.1);TuWRI1:乌拉图小麦 (EMS52893.1); GhWRI1:陆地棉(NP_001314053.1);VmWRI1:皱果桐(APQ47387.1)。Figure 6. Phylogenetic analysis of ElWRI1 among different plant speciesRcWRI1: Ricinus communis (NP_001310691.1); AtWRI1: Arabidopsis thaliana (OAP01705.1); JcWRI1: Jatropha curcas (AIR74897.1); HbWRI1: Hevea brasiliensis (XP_021674385.1); ZmWRI1: Zea mays (ONM19989.1); BnWRI1: Brassica napus (ADO16346.1); CsWRI1: Clycine soja (KHN10130.1); VvWRI1: Vitis vinifera (XP_010659009.1); CaWARI: Capsicum annuum (KAF3665339.1); CiWRI1: Carya illinoinensis (QQP23243.1); CnWRI1: Cocos nucifera (KAG1365549.1); TuWRI1: Triticum urartu (EMS52893.1); GhWRI1: Gossypium hirsutum (NP_001314053.1); VmWRI1: Vernicia montana (APQ47387.1).
图 6 不同物种WRI1蛋白的系统发育分析RcWRI1:蓖麻 (NP_001310691.1);AtWRI1:拟南芥 (OAP01705.1);JcWRI1:麻风树 (AIR74897.1);HbWRI1:巴西橡胶树 (XP_021674385.1);ZmWRI1:玉米 (ONM19989.1);BnWRI1:甘蓝型油菜 (ADO16346.1);CsWRI1:大豆 (KHN10130.1);VvWRI1:葡萄 (XP_010659009.1); CaWARI:辣椒(KAF3665339.1); CiWRI1:山核桃 (QQP23243.1); CnWRI1: 椰子 (KAG1365549.1);TuWRI1:乌拉图小麦 (EMS52893.1); GhWRI1:陆地棉(NP_001314053.1);VmWRI1:皱果桐(APQ47387.1)。Figure 6. Phylogenetic analysis of ElWRI1 among different plant speciesRcWRI1: Ricinus communis (NP_001310691.1); AtWRI1: Arabidopsis thaliana (OAP01705.1); JcWRI1: Jatropha curcas (AIR74897.1); HbWRI1: Hevea brasiliensis (XP_021674385.1); ZmWRI1: Zea mays (ONM19989.1); BnWRI1: Brassica napus (ADO16346.1); CsWRI1: Clycine soja (KHN10130.1); VvWRI1: Vitis vinifera (XP_010659009.1); CaWARI: Capsicum annuum (KAF3665339.1); CiWRI1: Carya illinoinensis (QQP23243.1); CnWRI1: Cocos nucifera (KAG1365549.1); TuWRI1: Triticum urartu (EMS52893.1); GhWRI1: Gossypium hirsutum (NP_001314053.1); VmWRI1: Vernicia montana (APQ47387.1).2.6 续随子ElWRI1表达分析
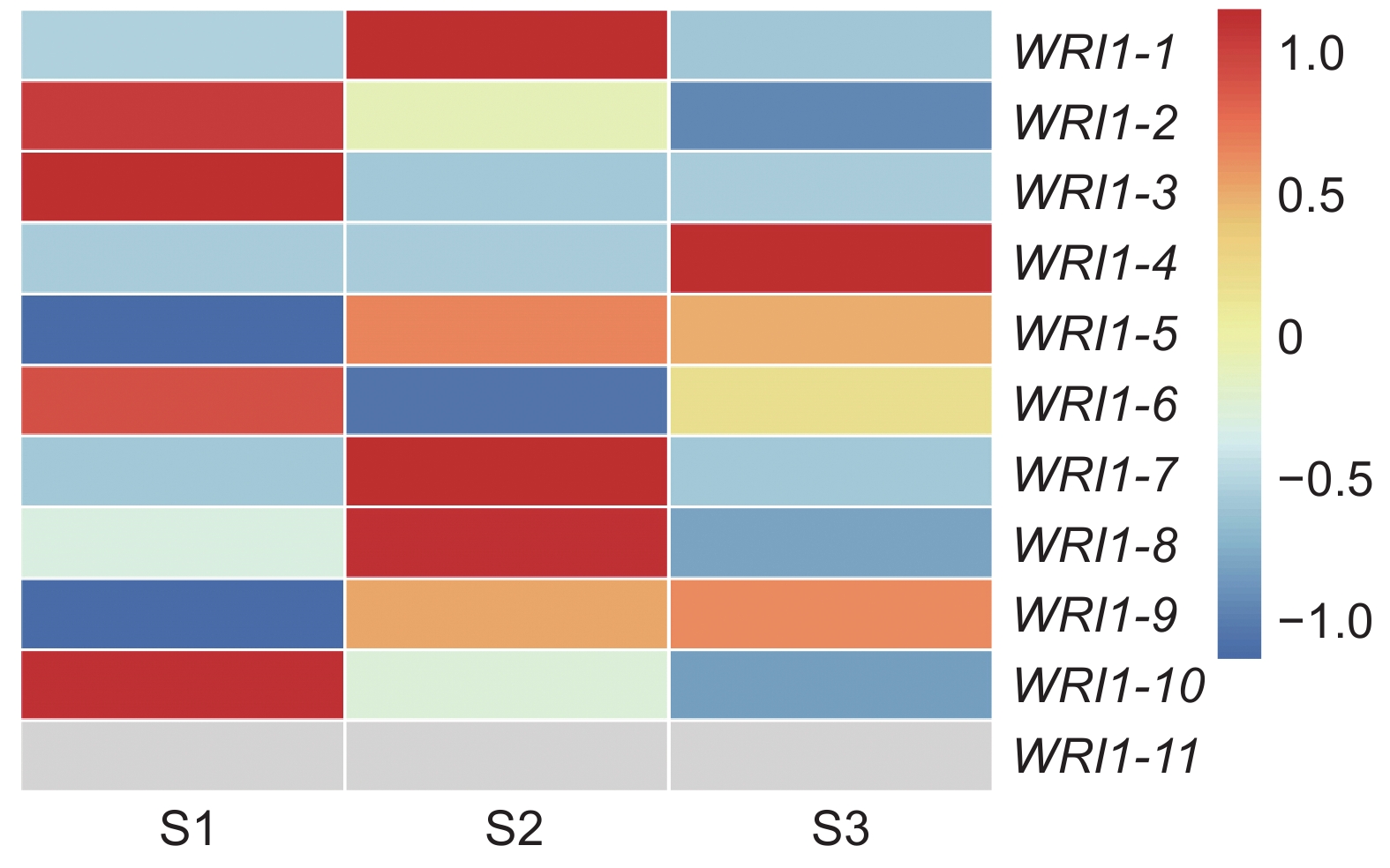
利用课题组已有的转录组数据,构建种子不同发育时期续随子ElWRI1的表达谱热图(图7)。样品来自种子发育的3个不同时期(S1、S2和S3)。除ElWRI1-5、ElWRI1-9和ElWRI1-11全程表达量偏低外,ElWRI1-2、ElWRI1-3、ElWRI1-6和ElWRI1-10在S1时期表达量最高;ElWRI1-1、ElWRI1-7和ElWRI1-8在S2时期表达量最高;而ElWRI1-4在S3时期表达量最高。
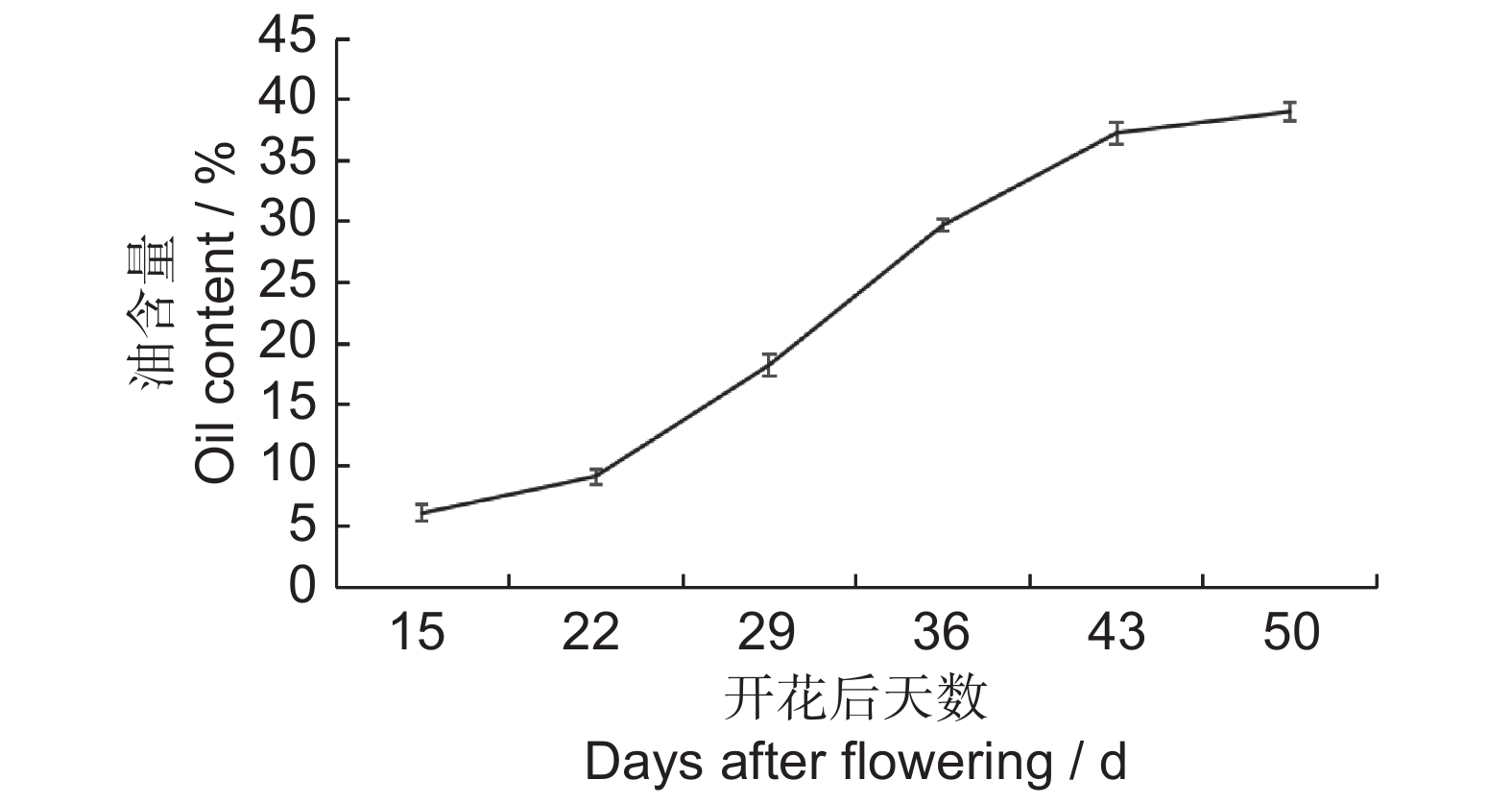
分析续随子种子不同发育时期含油率的变化(图8),发现花后22~43 d是续随子种子油脂积累的关键时期。因此,选择在S2时期表达量达到最高的WRI1-8进行下一步实验。
2.7 续随子ElWRI1-8基因表达载体的构建及烟草叶片的瞬时表达
为探究续随子ElWRI1-8基因对油脂合成的影响,以S2时期(开花后30 d)的cDNA为模板克隆续随子ElWRI1-8基因,并构建获得植物表达载体pCAMBIA1303-ElWRI1-8。
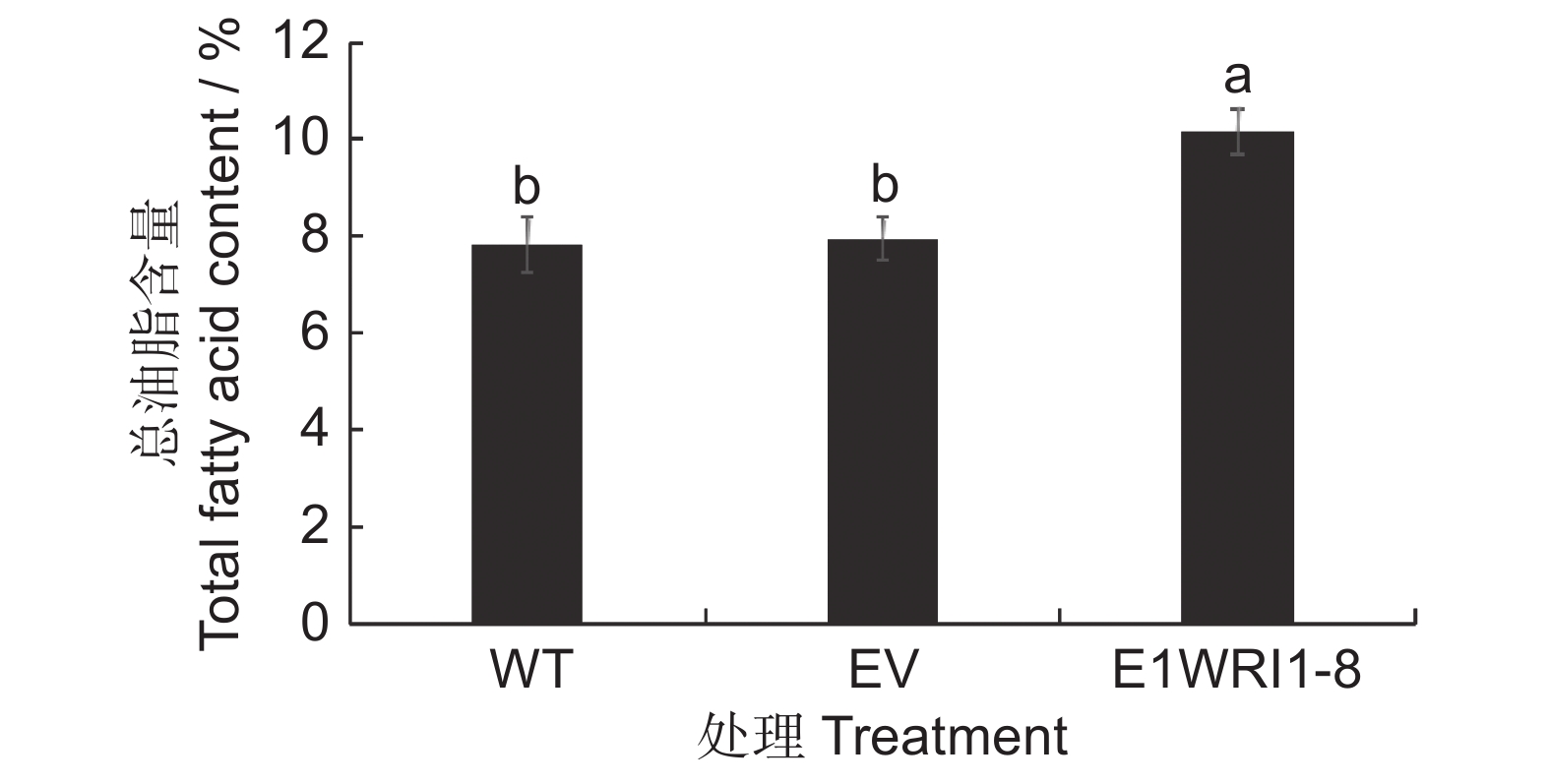
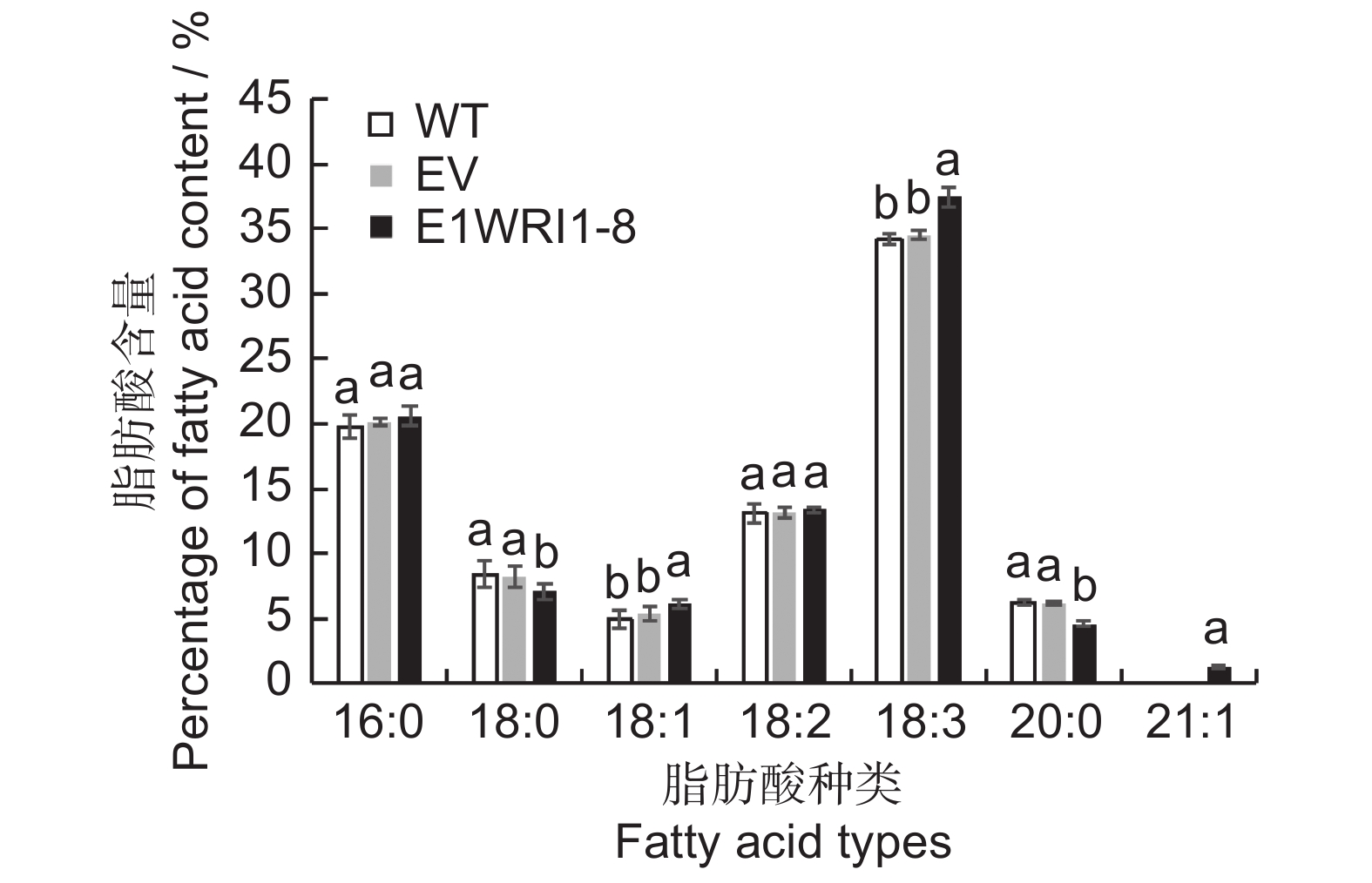
将含有目的基因及空载体质粒转入烟草叶片中进行瞬时表达,分析ElWRI1-8对烟草叶片总脂肪酸含量以及脂肪酸成分变化的影响(图9、图10)。总油脂含量检测结果如图9所示,与野生型(WT)叶片相比,转入空载体(EV)后总油脂含量几乎不变,而过表达ElWRI1-8后总脂肪酸含量增加显著,由7.83%上升至10.16%,是野生型的1.3倍。说明瞬时表达续随子ElWRI1-8基因后,可以在一定程度上提高烟草叶片的总油脂含量。脂肪酸组分测定结果显示(图10),转入基因ElWRI1-8后,脂肪酸16∶0和18∶2的变化不明显,脂肪酸18∶1和18∶3的含量比野生型及转空载有所上升,其中18∶1含量上升19.60%,18∶3含量上升8.90%。脂肪酸18∶0和20∶0的含量比野生型及转空载有所下降,前者下降14.97%,后者下降26.08%。瞬时表达后检测到一种新的长链脂肪酸22∶1,表明ElWRI1-8的表达会导致烟草叶片脂肪酸组分发生变化,且在一定程度上促进长链脂肪酸的合成。
3. 讨论
WRI1转录因子主要在油脂积累、糖酵解以及胚胎发育中起调控作用,WRI1最早在拟南芥突变体中被发现,并在随后分离克隆到AtWRI1基因。目前已在多种植物中进行了WRI1基因的克隆和表达研究[19]。
选择性剪切是调控基因表达的一种方式,至少有42%的拟南芥基因是选择性剪切的[20]。Ma等[21]研究发现,拟南芥AtWRI1有多种剪切形式,但只有剪接形式3(At3g54320.3)存在于拟南芥的多个组织和器官中。本文以AtWRI1(At3g54320.3)为索引,在续随子基因组中鉴定得到11个ElWRI1基因,并根据其在染色体上的位置分别命名为ElWRI1-1~ElWRI1-11。
多序列比对结果显示,除ElWRI1-1和ElWRI1-11之外,其余的ElWRI1蛋白序列均具有“VYL”氨基酸结构,这与An等[8]对拟南芥AtWRI1和亚麻荠CsWRI1的多序列比对结果一致。拟南芥AtWRI1第3外显子长度仅为9 bp,编码氨基酸“VYL”。这也符合An等[8]的研究结论,即大多数WRI1基因,包括AtWRI1,有7个外显子,其中第3个外显子仅包含9个核苷酸,且序列高度保守,编码WRI1蛋白的第1个AP2结构域的3个氨基酸残基(“VYL”)。Ma等[21]和Krizek等[22]的研究均表明,“VYL”中的1个或两个氨基酸的缺失,都会导致WRI1基因功能的降低或者缺失。
续随子11条ElWRI1序列都具有两个保守的AP2结构域,属AP2型转录因子。这与Riechmann等[23]提出的WRI1是一种AP2/乙烯反应元件结合蛋白(AP2/EREBP)型转录因子,这种转录因子至少有1个AP2保守结构域的结论一致。ElWRI1均为不稳定亲水蛋白,无跨膜结构,且均定位于细胞核,可能在细胞核中发挥作用。此外,ElWRI1-3、ElWRI1-6、ElWRI1-8、ElWRI1-10和ElWRI1-11存在信号肽,可能为分泌蛋白。表达分析结果显示,在续随子种子发育时期ElWRI1-8的表达量显著高于其他基因。由此推测,ElWRI1-8可能在续随子种子油脂合成积累过程中起着更为主导的调控作用。
为了进一步验证ElWRI1-8的功能,本研究通过农杆菌介导的侵染法将其转入烟草叶片中进行瞬时表达,并测定分析叶片中总脂肪酸含量以及脂肪酸组分。结果发现,与空载对照相比,转基因烟草叶片中的总脂肪酸含量显著升高,脂肪酸18∶1和18∶3的含量有所提高,脂肪酸18∶0和20∶0的含量则有所下降,此外,还检测到了对照中没有的长链脂肪酸22∶1。
综上所述,本研究鉴定获得了续随子ElWRI1-8基因,该基因具有AP2/EREBP型转录因子的典型特征,并在本氏烟草中通过异源过表达进行了功能验证。研究结果表明续随子ElWRI1-8基因具有提高植物油脂含量或改变植物脂肪酸成分的潜在应用价值。然而,过表达ElWRI1-8基因在提高烟草叶片油脂合成的同时,对淀粉、蛋白质和可溶性糖的合成是否产生影响,以及该基因主要调控哪些靶基因,还有待进一步研究。
-
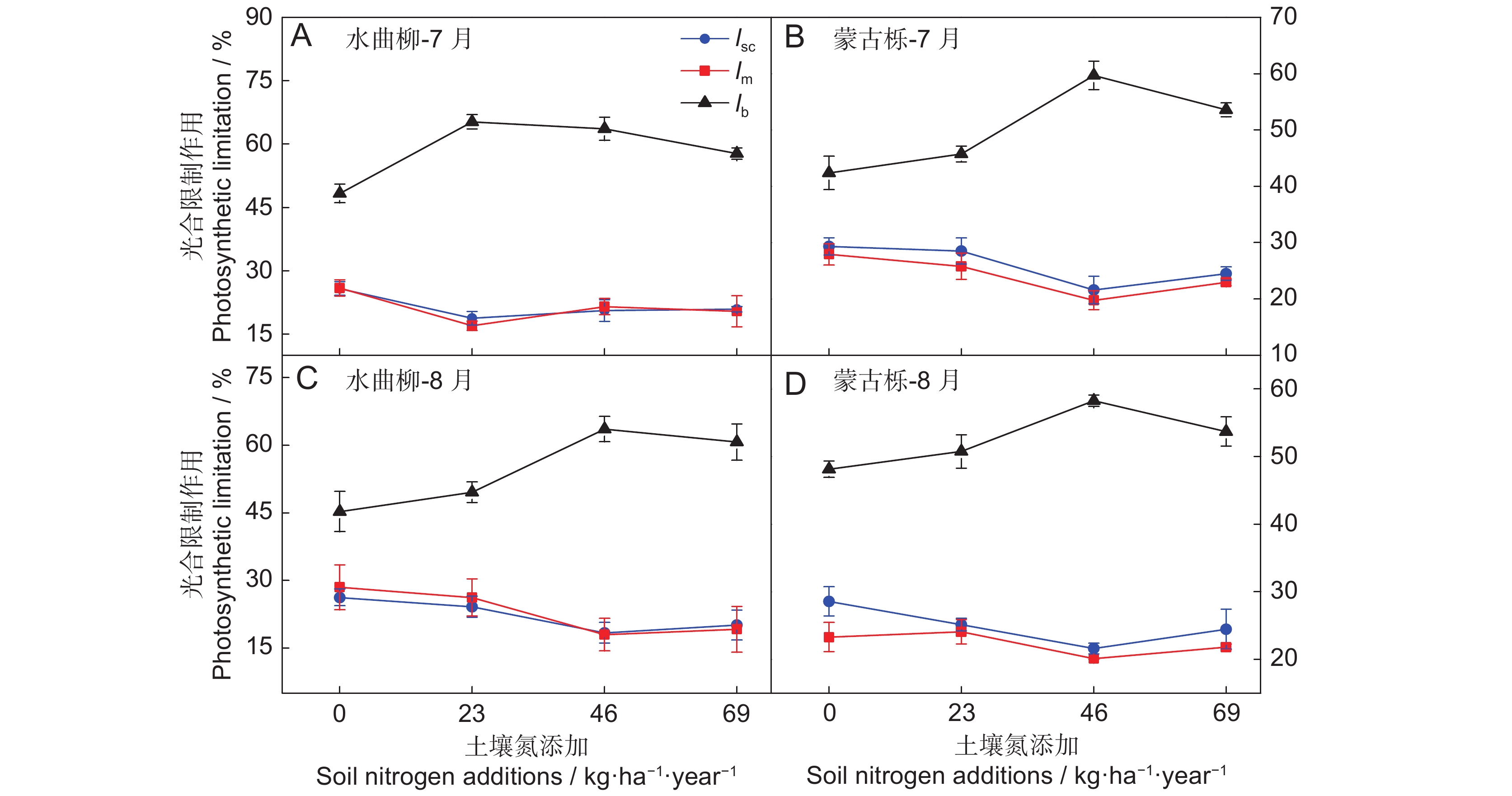
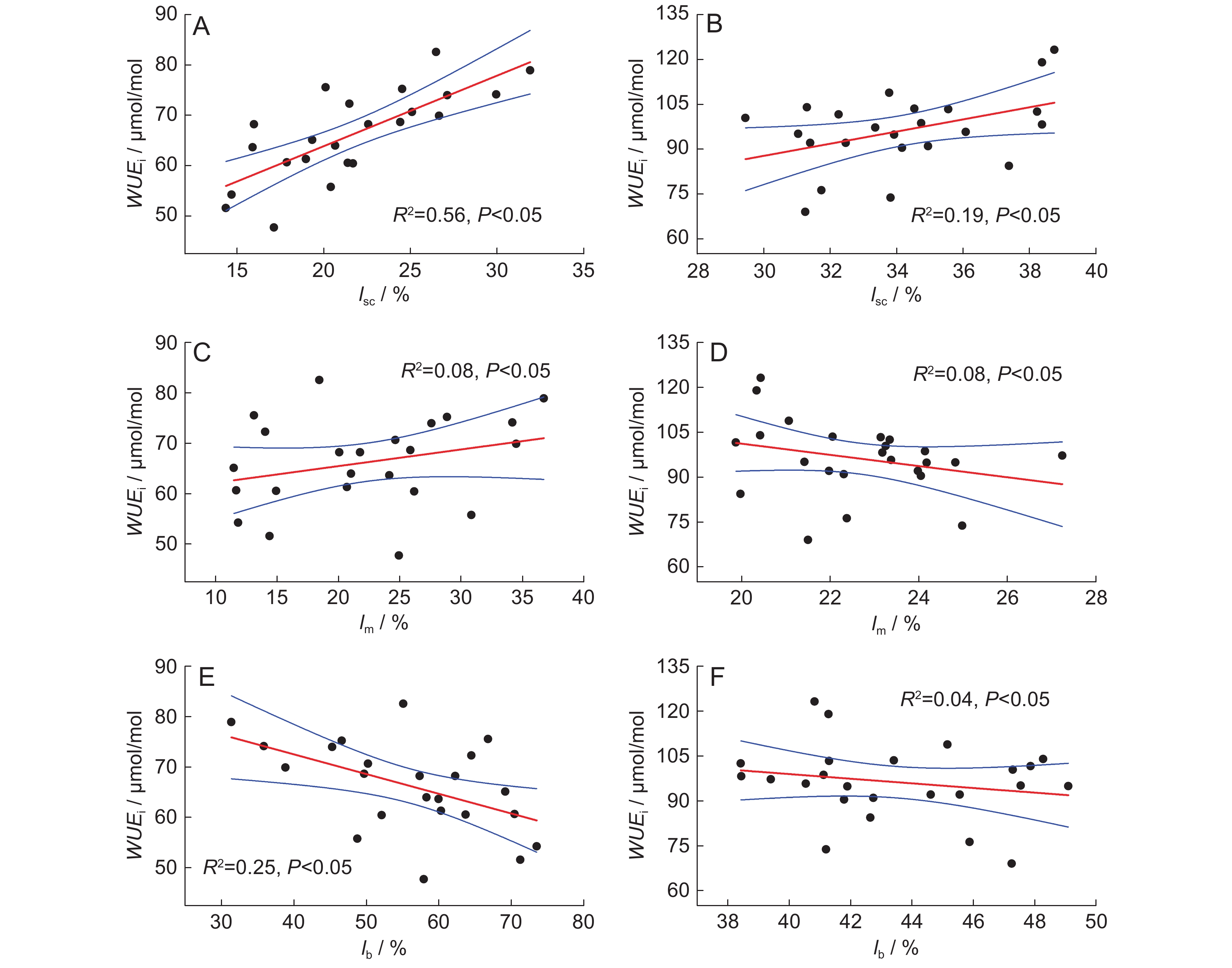
图 1 水曲柳(A、C)和蒙古栎(B、D)相对限制作用对土壤氮含量变化的响应
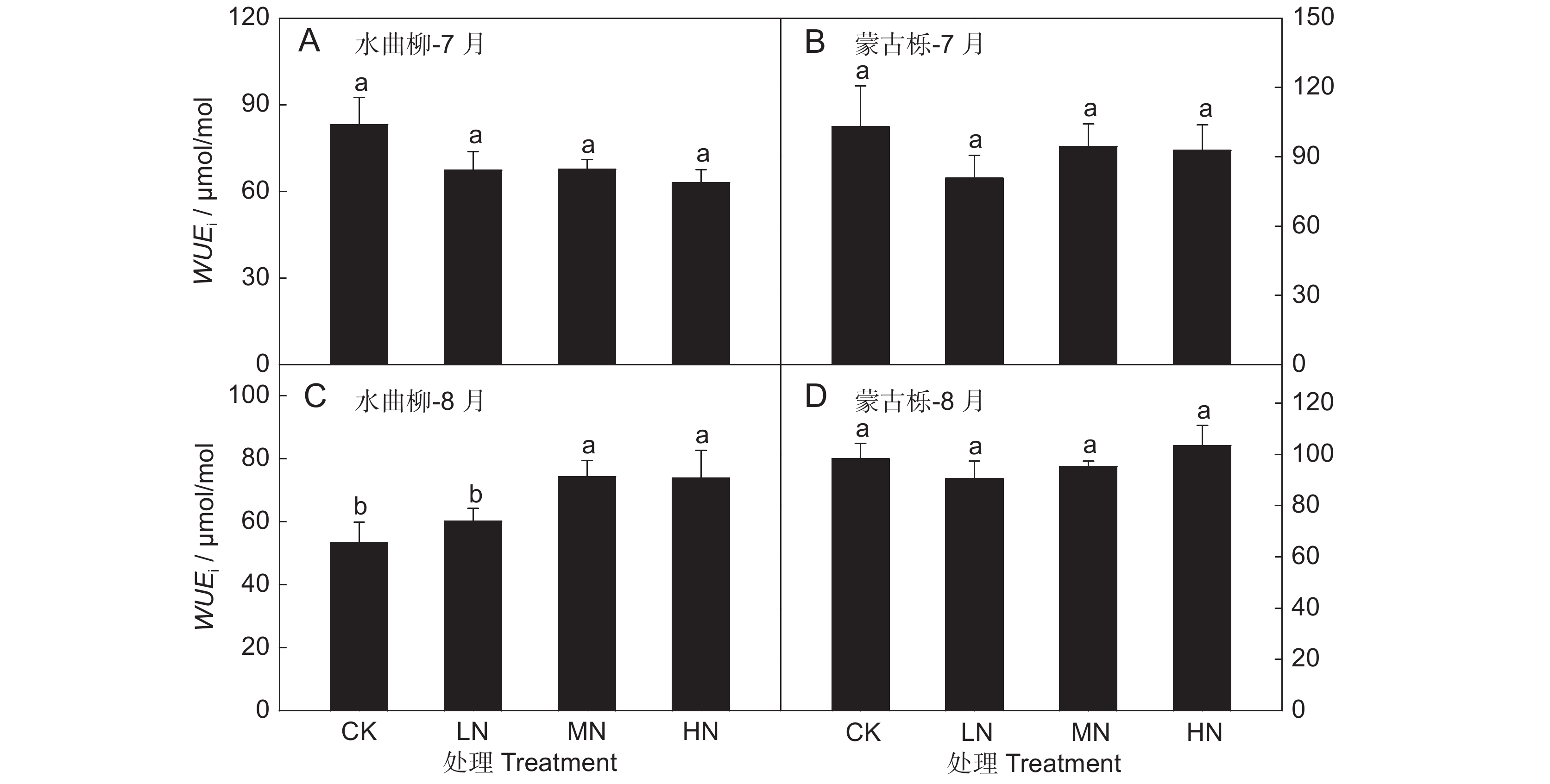
lsc为气孔限制,lm为叶肉限制,lb为生化限制。CK为对照(0 kg·ha−1·year−1),LN为低氮添加(23 kg·ha−1·year−1),MN为中氮添加(46 kg·ha−1·year−1),HN为高氮添加(69 kg·ha−1·year−1)。下同。
Figure 1. Reponses to relative limitations of soil nitrogen content in Fraxinus mandshurica (A, C) and Quercus mongolica (B, D) saplings
lsc, stomatal limitation; lm, mesophyll limitation; lb, biochemical limitation. CK, control (0 kg·ha−1·year−1); LN, low nitrogen addition (23 kg·ha−1·year−1); MN, medium nitrogen addition (46 kg·ha−1·year−1); HN, high nitrogen addition (69 kg·ha−1·year−1). Same below.
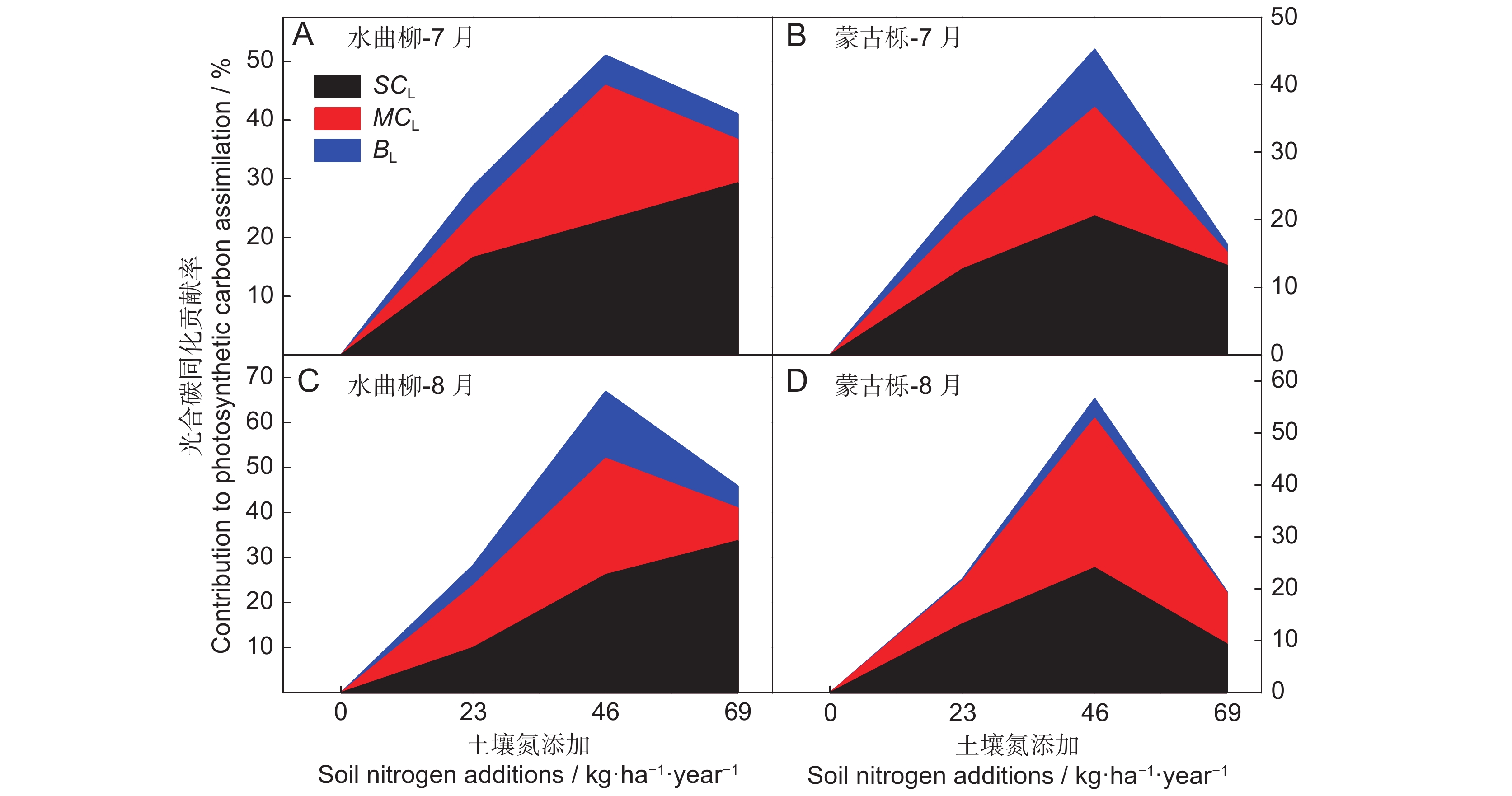
图 2 水曲柳(A、C)和蒙古栎(B、D)gsc、gm及生化能力对植株饱和光下碳同化相对贡献率随土壤氮含量的变化
SCL为gsc对叶片dPn/Pn的贡献值,MCL为gm对叶片dPn/Pn的贡献值,BL为生化能力对叶片dPn/Pn的贡献值。
Figure 2. Changes in contributions of gsc, gm, and biochemical capacity to light-saturated carbon assimilation (dPn/Pn) with soil nitrogen addition in Fraxinus mandshurica (A, C) and Quercus mongolica (B, D) saplings
SCL, contribution of gsc to dPn/Pn; MCL, contribution of gm to dPn/Pn; BL, contribution of biochemical capacity to dPn/Pn.
-
[1] Flexas J,Ribas-Carbó M,Diaz-Espejo A,Galmés J,Medrano H. Mesophyll conductance to CO2:current knowledge and future prospects[J]. Plant Cell Environ,2008,31 (5):602−621. doi: 10.1111/j.1365-3040.2007.01757.x
[2] Perez-Martin A,Michelazzo C,Torres-Ruiz JM,Flexas J,Fernández JE,et al. Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees:correlation with gene expression of carbonic anhydrase and aquaporins[J]. J Exp Bot,2014,65 (12):3143−3156. doi: 10.1093/jxb/eru160
[3] Han JM,Lei ZY,Flexas J,Zhang YJ,Carriquí M,et al. Mesophyll conductance in cotton bracts:anatomically determined internal CO2 diffusion constraints on photosynthesis[J]. J Exp Bot,2018,69 (22):5433−5443.
[4] Zhu K,Yuan FH,Wang AZ,Yang H,Guan DX,et al. Effects of soil rewatering on mesophyll and stomatal conductance and the associated mechanisms involving leaf anatomy and some physiological activities in Manchurian ash and Mongolian oak in the Changbai Mountains[J]. Plant Physiol Biochem,2019,144:22−34. doi: 10.1016/j.plaphy.2019.09.025
[5] Liu ZJ,Dickmann DI. Effects of water and nitrogen interaction on net photosynthesis,stomatal conductance,and water use-efficiency in two hybrid poplar clones[J]. Physiol Plant,1996,97 (3):507−512. doi: 10.1111/j.1399-3054.1996.tb00510.x
[6] Yamori W,Nagai T,Makino A. The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species[J]. Plant Cell Environ,2011,34 (5):764−777. doi: 10.1111/j.1365-3040.2011.02280.x
[7] Li Y,Ren BB,Yang XX,Xu GH,Shen QR,Guo SW. Chloroplast downsizing under nitrate nutrition restrained mesophyll conductance and photosynthesis in rice (Oryza sativa L. ) under drought conditions[J]. Plant Cell Physiol,2012,53 (5):892−900. doi: 10.1093/pcp/pcs032
[8] Xiong DL,Liu X,Liu LM,Douthe C,Li Y,et al. Rapid responses of mesophyll conductance to changes of CO2 concentration,temperature and irradiance are affected by N supplements in rice[J]. Plant Cell Environ,2015,38 (12):2541−2550. doi: 10.1111/pce.12558
[9] Eller F,Jensen K,Reisdorff C. Nighttime stomatal conductance differs with nutrient availability in two temperate floodplain tree species[J]. Tree Physiol,2017,37 (4):428−440.
[10] Zhu K,Wang AZ,Wu JB,Yuan FH,Guan DX,et al. Effects of nitrogen additions on mesophyll and stomatal conductance in Manchurian ash and Mongolian oak[J]. Sci Rep,2020,10:10038. doi: 10.1038/s41598-020-66886-x
[11] Galloway JN,Dentener FJ,Capone DG,Boyer EW,Howarth RW,et al. Nitrogen cycles:past,present,and future[J]. Biogeochemistry,2004,70 (2):153−226. doi: 10.1007/s10533-004-0370-0
[12] Zheng XH,Fu CB,Xu XK,Yan XD,Huang Y,et al. The Asian nitrogen cycle case study[J]. AMBIO,2002,31 (2):79−87. doi: 10.1579/0044-7447-31.2.79
[13] Loriaux SD,Avenson TJ,Welles JM,Mcdermitt DK,Eckles RD,et al. Closing in on maximum yield of chlorophyll fluorescence using a single multiphase flash of sub-saturating intensity[J]. Plant Cell Environ,2013,36 (10):1755−1770. doi: 10.1111/pce.12115
[14] Genty B,Briantais JM,Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence[J]. Biochim Biophys Acta Gen Subj,1989,990 (1):87−92. doi: 10.1016/S0304-4165(89)80016-9
[15] Valentini R,Epron D,Angelis P,Matteucci G,Dreyer E. In situ estimation of net CO2 assimilation,photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L. ) leaves:diurnal cycles under different levels of water supply[J]. Plant Cell Environ,1995,18 (6):631−640. doi: 10.1111/j.1365-3040.1995.tb00564.x
[16] Harley PC,Loreto F,Di Marco G,Sharkey TD. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2[J]. Plant Physiol,1992,98 (4):1429−1436. doi: 10.1104/pp.98.4.1429
[17] Walker BJ,Skabelund DC,Busch FA,Ort DR. An improved approach for measuring the impact of multiple CO2 conductances on the apparent photorespiratory CO2 compensation point through slope-intercept regression[J]. Plant Cell Environ,2016,39 (6):1198−1203. doi: 10.1111/pce.12722
[18] Grassi G,Magnani F. Stomatal,mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees[J]. Plant Cell Environ,2005,28 (7):834−849. doi: 10.1111/j.1365-3040.2005.01333.x
[19] Wang XX,Du TT,Huang JL,Peng SB,Xiong DL. Leaf hydraulic vulnerability triggers the decline in stomatal and mesophyll conductance during drought in rice[J]. J Exp Bot,2018,69 (16):4033−4045. doi: 10.1093/jxb/ery188
[20] Flexas J,Barón M,Bota J,Ducruet JM,Gallé A,et al. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris)[J]. J Exp Bot,2009,60 (8):2361−2377. doi: 10.1093/jxb/erp069
[21] Cano FJ,López R,Warren CR. Implications of the mesophyll conductance to CO2 for photosynthesis and water-use efficiency during long-term water stress and recovery in two contrasting Eucalyptus species[J]. Plant Cell Environ,2014,37 (11):2470−2490. doi: 10.1111/pce.12325
[22] Von Caemmerer S,Evans JR,Hudson GS,Andrews TJ. The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco[J]. Planta,1994,195:88−97. doi: 10.1007/BF00206296
[23] Osmond CB, Björkman O, Anderson DJ. Physiological Processes in Plant Ecology: Toward a Synthesis with Atriplex[M]. Berlin: Springer, 1980: 468.
[24] Bown HE,Watt MS,Mason EG,Clinton PW,Whitehead D. The influence of nitrogen and phosphorus supply and genotype on mesophyll conductance limitations to photosynthesis in Pinus radiata[J]. Tree Physiol,2009,29 (9):1143−1151. doi: 10.1093/treephys/tpp051
[25] Li Y,Gao YX,Xu XM,Shen QR,Guo SW. Light-saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L. ) leaves is related to chloroplastic CO2 concentration[J]. J Exp Bot,2009,60 (8):2351−2360. doi: 10.1093/jxb/erp127
[26] Galmés J,Medrano H,Flexas J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms[J]. New Phytol,2007,175 (1):81−93. doi: 10.1111/j.1469-8137.2007.02087.x
[27] Wang XX,Wang WC,Huang JL,Peng SB,Xiong DL. Diffusional conductance to CO2 is the key limitation to photosynthesis in salt-stressed leaves of rice (Oryza sativa)[J]. Physiol Plantarum,2018,163 (1):45−58. doi: 10.1111/ppl.12653
[28] Zhu LL,Li HC,Thorpe MR,Hocart CH,Song X. Stomatal and mesophyll conductance are dominant limitations to photosynthesis in response to heat stress during severe drought in a temperate and a tropical tree species[J]. Trees,2021,35 (5):1613−1626. doi: 10.1007/s00468-021-02140-9
[29] 张继澍, 胡景江, 王玉国, 张少英, 宋纯鹏. 植物生理学[M]. 北京: 高等教育出版社, 2006: 112-120. [30] Perez-Martin A,Torres-Ruiz JM,Flexas J,Fernández JE,Diaz-Espejo A. Physiological and genetic response of olive leaves to water stress and recovery:implications of mesophyll conductance and genetic expression of aquaporins and carbonic anhydrase[J]. Acta Hortic,2011,922 (922):99−105.
[31] Zhu K,Yuan FH,Wang AZ,Wu JB,Guan DX,et al. Stomatal,mesophyll and biochemical limitations to soil drought and rewatering in relation to intrinsic water-use efficiency in Manchurian ash and Mongolian oak[J]. Photosynthetica,2021,59 (1):49−60. doi: 10.32615/ps.2020.084
[32] Barbour MM,Kaiser BN. The response of mesophyll conductance to nitrogen and water availability differs between wheat genotypes[J]. Plant Sci,2016,251:119−127. doi: 10.1016/j.plantsci.2016.03.012
[33] Tomás M,Medrano H,Brugnoli E,Escalona JM,Martorell S,et al. Variability of mesophyll conductance in grapevine cultivars under water stress conditions in relation to leaf anatomy and water use efficiency[J]. Aust J Grape Wine Res,2014,20 (2):272−280. doi: 10.1111/ajgw.12069
[34] 蒋高明,何维明. 毛乌素沙地若干植物光合作用、蒸腾作用和水分利用效率种间及生境间差异(英文)[J]. 植物学报,1999,41(10):1114−1124. Jiang GM,He WM. Species- and habitat-variability of photosynthesis,transpiration and water use efficiency of different plant species in Maowusu sand area[J]. Acta Botanica Sinica,1999,41 (10):1114−1124.
[35] Sáez PL,Galmés J,Ramírez CF,Poblete L,Rivera BK,et al. Mesophyll conductance to CO2 is the most significant limitation to photosynthesis at different temperatures and water availabilities in Antarctic vascular species[J]. Environ Exp Bot,2018,156:279−287. doi: 10.1016/j.envexpbot.2018.09.008
[36] Aranda I,Rodríguez-Calcerrada J,Robson TM,Cano FJ,Alté L,Sánchez-Gómez D. Stomatal and non-stomatal limitations on leaf carbon assimilation in beech (Fagus sylvatica L. ) seedlings under natural conditions[J]. For Syst,2012,21 (3):405−417.
-
期刊类型引用(1)
1. 惠生娟,葛丽萍,王子瑜,张玉胜,苏云婷,孙岩,李润植. 续随子MYB基因家族的鉴定及ElMYB114在油脂合成中的功能分析. 植物科学学报. 2025(01): 92-101 .  本站查看
本站查看
其他类型引用(0)



 下载:
下载: