Research progress of UbiA membrane-bound aromatic prenyltransferases in plants
-
摘要:
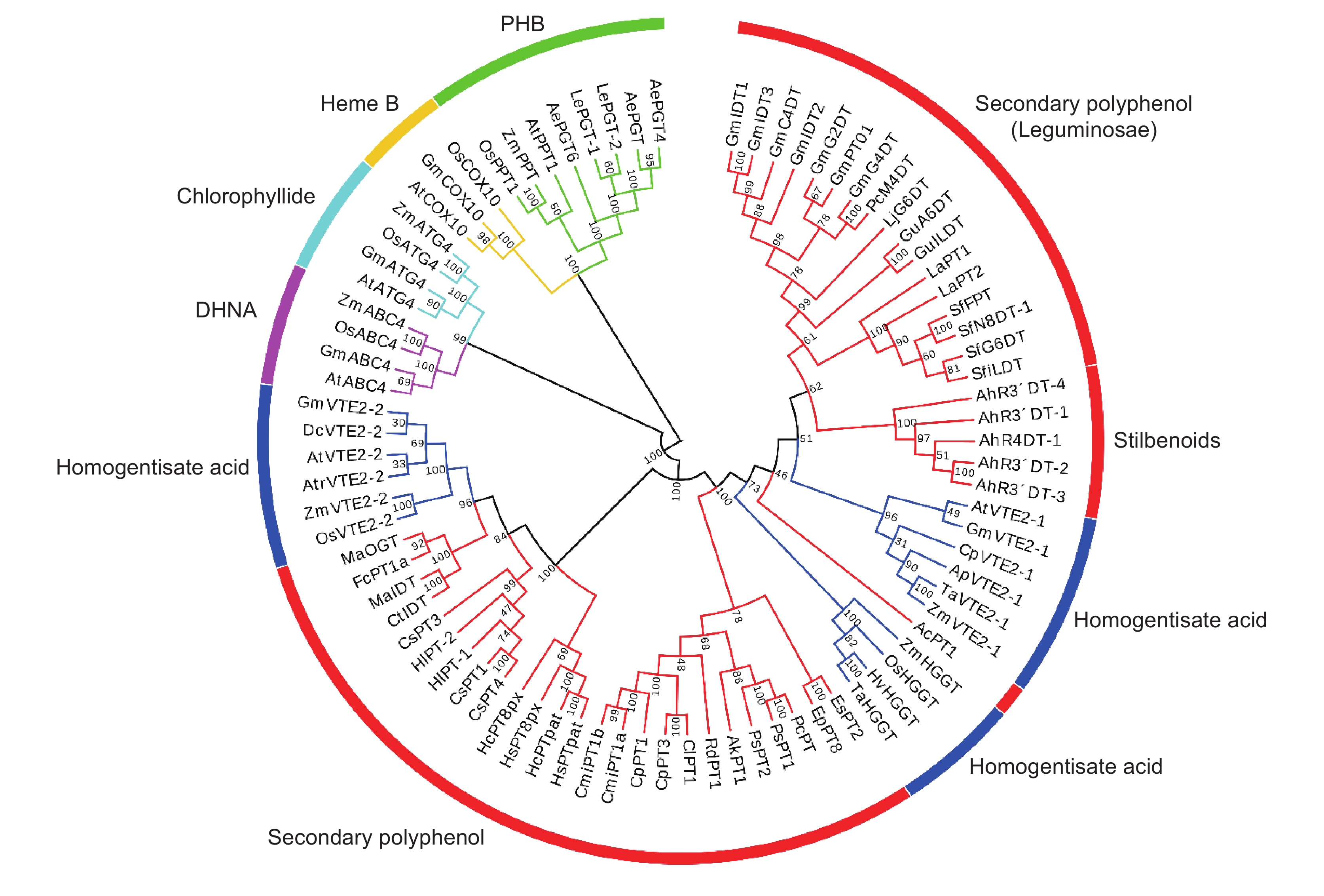
UbiA膜结合型芳香族异戊烯基转移酶(Prenyltransferases,PT)可催化异戊烯基单元转移到芳香族母核上形成C-C(或C-O)键,在植物中参与合成重要的代谢产物,如泛醌、质体醌、叶绿素、生育酚等。植物中多种具有异戊烯基的芳香族次生代谢物也是该类酶作用的产物。异戊烯基的引入增加了天然产物结构多样性和生物活性。本文介绍了植物中UbiA家族的基本类型,归纳了57个已鉴定功能的与次生代谢物(类黄酮、香豆素、二苯乙烯等)合成相关的UbiA PTs底物选择性、催化特点及其与初生代谢相关PTs的系统发育关系,并对异戊烯基转移酶基因的挖掘策略,以及利用微生物代谢工程定向合成活性异戊烯基化合物的应用前景进行了展望。
Abstract:UbiA membrane-bound aromatic prenyltransferases (UbiA PTs) catalyze the transfer of prenyl moieties to aromatic acceptor molecules to form C-C or C-O bonds, and participate in the biosynthesis of important plant chemicals, including ubiquinone, plastoquinone, chlorophyll, and tocopherol. A variety of aromatic secondary metabolites with prenyl groups in plants are also products of this class of enzyme. The introduction of prenyl groups increases the structural diversity and biological activity of natural products. In this paper, we introduce the basic types of UbiA families in plants, summarize the substrate selectivity and catalytic characteristics of 57 UbiA PTs related to biosynthesis of secondary metabolites (flavonoids, coumarins, stilbenes), and discuss their phylogenetic relationship with primary metabolism-related PTs. We also discuss the exploration strategies of prenyltransferase genes and the application prospects of targeted synthesis of active prenylated compounds by microbial metabolic engineering.
-
Keywords:
- Prenyltransferases /
- Primary metabolism /
- Secondary metabolism /
- Biosynthesis
-
1 1)如需查阅附表内容请登录《植物科学学报》网站(http://www.plantscience.cn)查看本期文章。2 1)如需查阅附表内容请登录《植物科学学报》网站(http://www.plantscience.cn)查看本期文章。 -
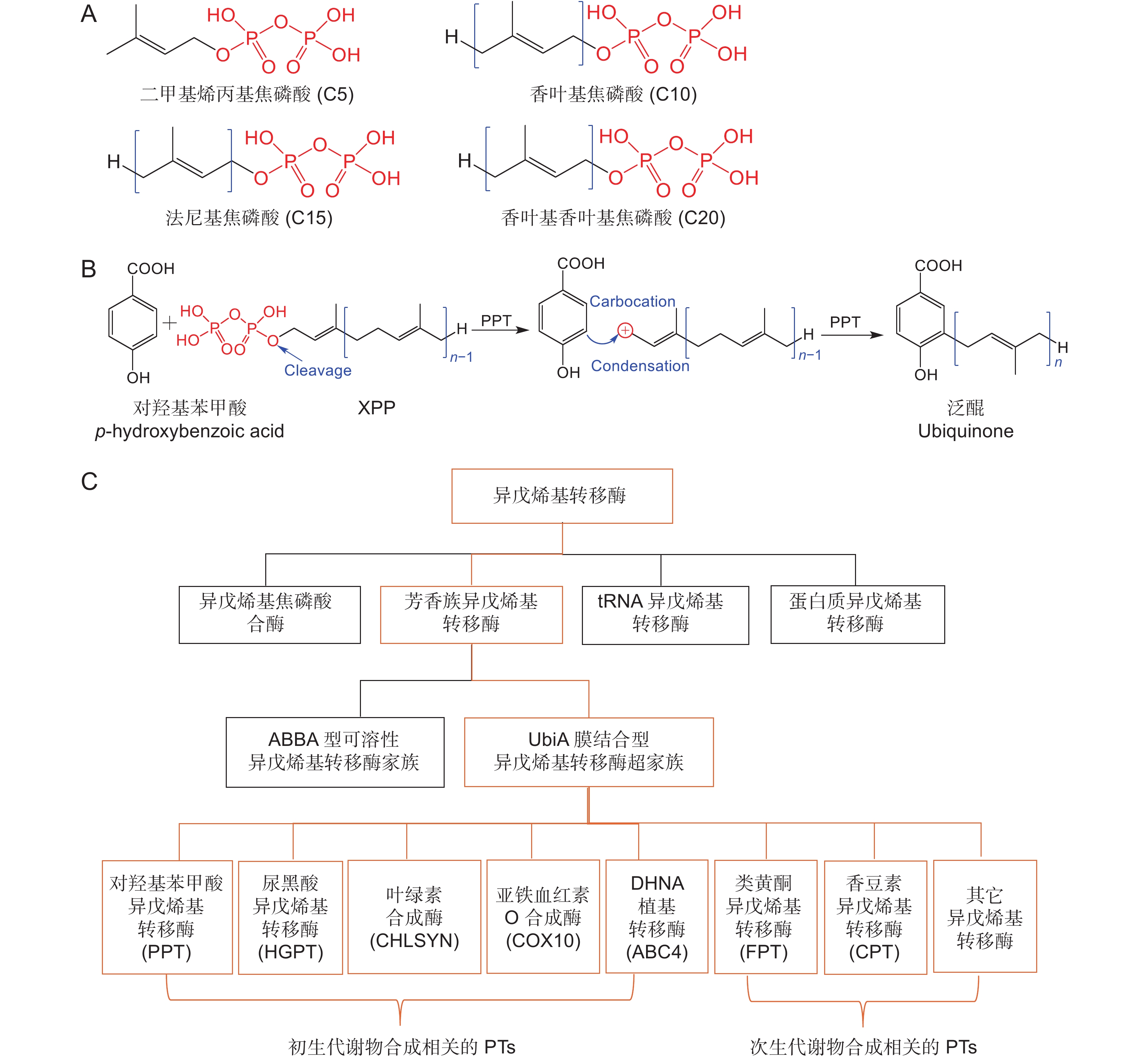
图 1 UbiA异戊烯基转移酶的分类、一般催化机制和供体的结构式
A:异戊二烯焦磷酸结构式;B:UbiA异戊烯基转移酶的一般催化机制;C:异戊烯基转移酶的分类。
Figure 1. Classification and general catalytic mechanism of UbiA prenyltransferases and structural formula of isoprenyl diphosphates
A: Structural formula of isoprenyl diphosphates; B: General catalytic mechanism of UbiA superfamily prenyltransferases; C: Classification of prenyltransferases.
表 1 植物类黄酮异戊烯基转移酶的催化特性
Table 1 Catalytic properties of flavonoid prenyltransferases in plants
物种名称
Species name蛋白名称
Protein name底物类型
Substrate type底物名称
Substrate name异戊烯基供体
Prenyl donor异戊烯基取代位点
Prenyl substitution site二价阳离子
Divalent cation参考文献
References苦参
Sophora flavescens Alt.SfN8DT-1 二氢黄酮 Liquiritigenin>Naringenin>
HesperetinDMAPP A环C-8位 Mg2 + [7] SfiLDT 查尔酮 Isoliquiritigenin DMAPP 未知 Mg2 + [33] SfG6DT 异黄酮 Genistein>Biochanina DMAPP、GPP1、FPP1 A环C-6位 Mg2 + >Ni2 + >
Mn2 + >Ca2 +[33] SfFPT 二氢查尔酮 Phloretin DMAPP、GPP2 A环C-3'位 Mg2 + >Ba2 + >Ca2 + >Fe2 + >Co2 + >Cu2 + >Zn2 + >Mn2 + [28] 二氢黄酮 Eriodictyol>Naringenin>
Pinocembrin>Liquiritigenin>
Hesperetin>Isosakuranetin>
Steppogenin>Tsugafolin>
SakuranetinA环C-8位 黄酮 Chrysin A环C-8位 二氢黄酮醇 Taxifolin A环C-8位 大豆
Glycine max (L.) Merr.GmG4DT 紫檀烷 Glycinol>Maackiain DMAPP A环C-4位 Mg2 + >Mn2 + >Co2 + [30] GmG2DT 紫檀烷 Glycinol DMAPP A环C-2位 Mg2 + >Mn2 + [29] GmPT01 紫檀烷 Glycinol DMAPP A环C-2位 Mg2 + [31] GmIDT1 异黄酮 Daidzein>Genistein DMAPP B环 Mg2 + >Mn2 + [29] GmIDT2 异黄酮 Daidzein≈Genistein DMAPP A环 Mg2 + >Mn2 + [29] GmIDT3 异黄酮 Daidzein、Genistein DMAPP 未知 Mg2 + [31] 白羽扇豆
Lupinus albus L.LaPT1 异黄酮 2-Hydroxygenistein>
GenisteinDMAPP B环C-3'位 Mg2 + >Mn2 + >Ni2 + >Co2 + >Zn2 + >Ca2 + [34] LaPT2 黄酮醇 Kaempferol>Kaempferide>Quercetin>Galangin>
Fesitin>MorinDMAPP A环C-8位 Mg2 + [12] 二氢黄酮 Naringenin DMAPP 未知 甘草
Glycyrrhiza uralensis Fisch.GuA6DT 黄酮 Apigenin>Chrysin>
Diosmtin>Luteolin>
Norartocarpetin>
ChrysoeroilDMAPP、GPP1 A环C-6位 Mg2 + >Mn2 + >Zn2 + >Fe2 + >Co2 + >Ca2 + >Ba2 + [11] GuILDT 查尔酮 2',4'-Dihydroxychalcone>
Isoliquiritigenin>
2,4,2',4'-Tetrahydro-xychalcone>
NaringeninchalconeDMAPP A环C-3'位 Mg2 + >Co2 + >Ni2 + >Fe2 + >Ba2 + >Mn2 + >Ca2 + [35] 百脉根
Lotus japonicus L.LjG6DT 异黄酮 Genistein DMAPP A环C-6位 Mg2 + >Co2 + >Mn2 + >Ca2 + >Zn2 + >Fe2 + [36] 补骨脂
Psoralea corylifolia (L.) Medik.PcM4DT 紫檀烷 Maackiain>3-Hydroxy-9-methoxypterocarpan DMAPP A环C-4位 Mg2 + >Mn2 + >Co2 + >Fe2 + >Ba2 + >Sr2 + >Ca2 + >Sn2 + >Ni2 + >Zn2 + [37] 桑
Morus alba L.MaIDT 查尔酮 Isoliquiritigenin>
2',4'-Dihydroxychalcone>
2,4,2',4'-Tetrahydroxychalcone>
ButeinDMAPP、GPP1 A环C-3'位 Mg2 + >Ba2 + >Ca2 + >Mn2 + >Fe2 + >Ni2 + [32] 异黄酮 Genistein>2'-Hydroxygenistein A环C-6位 黄酮 Apigenin A环C-6位 柘树
Cudrania tricuspidata (Carr.) Bur.CtIDT 查尔酮 Isoliquiritigenin>
2,4,2',4'-Tetrahydroxychalcone>
2',4'-Dihydroxychalcone>
ButeinDMAPP、GPP1 A环C-3'位 Mg2 + >Mn2 + >Ca2 + >Fe2 + >Ba2 + [32] 异黄酮 2'-Hydroxygenistein>
GenisteinA环C-6位 大麻
Cannabis sativa L.CsPT3 黄酮 Chrysoeriol>Apigenin DMAPP、GPP A环C-6位 Mg2 + [38] CsPT8 黄酮 Apigenin DMAPP 未知 Mg2 + [38] 啤酒花
Humulus lupulus L.HlPT-1 查尔酮 Naringenin chalcone DMAPP A环C-3'位 Mg2 + [39] 柔毛淫羊藿
Epimedium pubescens Maxim.EpPT8 黄酮醇 Kaempferol> Quercetin DMAPP A环C-8位 Mg2 + [40] 黄酮 Apigenin 箭叶淫羊藿
Epimedium sagittatum (Sieb. et Zucc.) Maxim.EsPT2 黄酮醇 Kaempferol>Kaempferide DMAPP A环C-8位 Mg2 + [41] 二氢黄酮 Naringenin 注:“>”用于表示对底物的催化活性顺序;1 研究只证明提供了该供体与最适底物发生异戊烯基化反应;2 GPP作为供体时,SfFPT仅催化pinocembrin,isosakuranetin和naringenin发生异戊烯基化反应。 Notes: “>” indicates order of catalytic activity to the substrate; 1 Prenylation of the donor with an optimal substrate is demonstrated; 2 SfFPT only catalyzed prenylation of pinocembrin, isosakuranetin, and naringenin when GPP was used as the prenyl donor. 表 2 植物中香豆素异戊烯基转移酶的催化特性
Table 2 Catalytic properties of coumarin prenyltransferases in plants
物种名称
Species
name蛋白名称
Protein name底物名称
Substrate name异戊烯基
供体
Prenyl donor异戊烯基取代位点
Prenyl substitution
site二价阳离子
Divalent cation参考文献
References欧芹Petroselinum crispum (Mill.) Hill PcPT Umbelliferone DMAPP C-6位>C-8位 Mg2 + [44] 欧防风Pastinaca sativa L. PsPT1 Umbelliferone DMAPP C-6位>C-8位 Mg2 + [42] PsPT2 Umbelliferone DMAPP C-8位>C-6位 Mg2 + [42] 柠檬Citrus limon (L.) Burm. F. ClPT1 Umbelliferone>Esculetin>5,7-hydroxycoumarin
>5-Methoxy-7-hydroxycoumarinGPP C-8位 Mg2 + [45] 无花果Ficus carica L. FcPT1 Umbelliferone DMAPP C-6位 Mg2 + [46] 5-Methoxy-7-hydroxycoumarin DMAPP 未知 Mg2 + 葡萄柚Citrus paradisi Macf. CpPT1 5,7-Dihydroxycoumarin, 8-Hydroxybergapten 5-Hydroxy-7-methoxycoumarin, Bergaptol, GPP 5-OH或8-OH Mg2 + >Ni2 + >Co2 + >Mn2 + >Zn2 + >Ca2 + [47] CpPT3 Umbelliferone GPP C-8位 Mg2 + [47] 小苦橙Citrus micrantha Wester CmiPT1a / b Bergaptol和Xanthotoxol GPP 5-OH或8-OH Mg2 + [47] 明日叶Angelica keiskei (Miquel) Koidz. AkPT1 Bergaptol和Xanthotoxol DMAPP 5-OH或8-OH Mg2 + >Mn2 + >Ca2 + [47] 大豆Glycine max (L.) Merr. GmC4DT Coumestrol DMAPP C-4位 Mg2 + >Mn2 + [29] 九里香Murraya exotica L. MePT1 Umbelliferone GPP C-8位、C-6位和7-OH Mg2 + [48] -
[1] Winkelblech J,Fan AL,Li SM. Prenyltransferases as key enzymes in primary and secondary metabolism[J]. Appl Microbiol Biotechnol,2015,99 (18):7379−7397. doi: 10.1007/s00253-015-6811-y
[2] Yang YH,Ke N,Liu SX,Li WK. Structural and biochemical analysis of intramembrane prenyltransferases in the UbiA superfamiIy[J]. Methods Enzymol,2017,584:309−347.
[3] Li WK. Bringing bioactive compounds into membranes:the UbiA superfamily of intramembrane aromatic prenyltransferases[J]. Trends Biochem Sci,2016,41 (4):356−370. doi: 10.1016/j.tibs.2016.01.007
[4] Bonitz T,Alva V,Saleh O,Lupas AN,Heide L. Evolutionary relationships of microbial aromatic prenyltransferases[J]. PLoS One,2011,6 (11):e27336. doi: 10.1371/journal.pone.0027336
[5] Young IG,Leppik RA,Hamilton JA,Gibson F. Biochemical and genetic studies on ubiquinone biosynthesis in Escherichia coli K-12:4-hydroxybenzoate octaprenyltransferase[J]. J Bacteriol,1972,110 (1):18−25. doi: 10.1128/jb.110.1.18-25.1972
[6] Wang J,Chu SS,Zhu Y,Cheng H,Yu DY. Positive selection drives neofunctionalization of the UbiA prenyltransferase gene family[J]. Plant Mol Biol,2015,87 (4-5):383−394. doi: 10.1007/s11103-015-0285-2
[7] Sasaki K,Mito K,Ohara K,Yamamoto H,Yazaki K. Cloning and characterization of naringenin 8-prenyltransferase,a flavonoid-specific prenyltransferase of Sophora flavescens[J]. Plant Physiol,2008,146 (3):1075−1084. doi: 10.1104/pp.107.110544
[8] Bo ST, Chang SK, Zhu H, Jiang YM, Yang B. Naturally occurring prenylated stilbenoids: food sources, biosynthesis, applications and health benefits[J]. Crit Rev Food Sci Nutr, 2022. Doi: 10.1080/10408398.2022.2056131.
[9] De Bruijn WJC,Levisson M,Beekwilder J,van Berkel WJH,Vincken JP. Plant aromatic prenyltransferases:tools for microbial cell factories[J]. Trends Biotechnol,2020,38 (8):917−934. doi: 10.1016/j.tibtech.2020.02.006
[10] Marin M,Manez S. Recent trends in the pharmacological activity of isoprenyl phenolics[J]. Curr Med Chem,2013,20 (2):272−279. doi: 10.2174/092986713804806676
[11] Li JH,Chen RD,Wang RS,Liu X,Xie D,et al. GuA6DT,a regiospecific prenyltransferase from Glycyrrhiza uralensis,catalyzes the 6-prenylation of flavones[J]. ChemBioChem,2014,15 (11):1673−1681. doi: 10.1002/cbic.201402160
[12] Liu JY,Xia YY,Jiang WB,Shen GA,Pang YZ. LaPT2 gene encodes a flavonoid prenyltransferase in white lupin[J]. Front Plant Sci,2021,12:673337. doi: 10.3389/fpls.2021.673337
[13] Okada K,Ohara K,Yazaki K,Nozaki K,Uchida N,et al. The AtPPT1 gene encoding 4-hydroxybenzoate polyprenyl diphosphate transferase in ubiquinone biosynthesis is required for embryo development in Arabidopsis thaliana[J]. Plant Mol Biol,2004,55 (4):567−577. doi: 10.1007/s11103-004-1298-4
[14] Ohara K,Yamamoto K,Hamamoto M,Sasaki K,Yazaki K. Functional characterization of OsPPT1,which encodes p-hydroxybenzoate polyprenyltransferase involved in ubiquinone biosynthesis in Oryza sativa[J]. Plant Cell Physiol,2006,47 (5):581−590. doi: 10.1093/pcp/pcj025
[15] Yazaki K,Kunihisa M,Fujisaki T,Sato F. Geranyl diphosphate:4-hydroxybenzoate geranyltransferase from Lithospermum erythrorhizon:cloning and characterization of a key enzyme in Shikonin biosynthesis[J]. J Biol Chem,2002,277 (8):6240−6246. doi: 10.1074/jbc.M106387200
[16] Ohara K,Muroya A,Fukushima N,Yazaki K. Functional characterization of LePGT1,a membrane-bound prenyltransferase involved in the geranylation of p-hydroxybenzoic acid[J]. Biochem J,2009,421 (2):231−241. doi: 10.1042/BJ20081968
[17] Wang S,Wang RS,Liu T,Zhan ZL,Kang LP,et al. Production of 3-geranyl-4-hydroxybenzoate acid in yeast,an important intermediate of Shikonin biosynthesis pathway[J]. FEMS Yeast Res,2017,17 (7):fox065.
[18] Venkatesh TV,Karunanandaa B,Free DL,Rottnek JM,Baszis SR,Valentin HE. Identification and characterization of an Arabidopsis homogentisate phytyltransferase paralog[J]. Planta,2006,223 (6):1134−1144. doi: 10.1007/s00425-005-0180-1
[19] Sadre R,Gruber J,Frentzen M. Characterization of homogentisate prenyltransferases involved in plastoquinone-9 and tocochromanol biosynthesis[J]. FEBS Lett,2006,580 (22):5357−5362. doi: 10.1016/j.febslet.2006.09.002
[20] Tian L,DellaPenna D,Dixon RA. The pds2 mutation is a lesion in the Arabidopsis homogentisate solanesyltransferase gene involved in plastoquinone biosynthesis[J]. Planta,2007,226 (4):1067−1073. doi: 10.1007/s00425-007-0564-5
[21] 姚兴兰,王磊,张兰. 植物维生素E生物强化研究进展[J]. 生物技术进展,2020,10(5):479−486. doi: 10.19586/j.2095-2341.2020.0046 Yao XL,Wang L,Zhang L. Progress of vitamin E biofortification in plants[J]. Current Biotechnology,2020,10 (5):479−486. doi: 10.19586/j.2095-2341.2020.0046
[22] Eckhardt U,Grimm B,Hörtensteiner S. Recent advances in chlorophyll biosynthesis and breakdown in higher plants[J]. Plant Mol Biol,2004,56 (1):1−14. doi: 10.1007/s11103-004-2331-3
[23] Hederstedt L. Heme A biosynthesis[J]. Biochim Biophys Acta,2012,1817 (6):920−927. doi: 10.1016/j.bbabio.2012.03.025
[24] Basset GJ,Latimer S,Fatihi A,Soubeyrand E,Block A. Phylloquinone (vitamin K1):occurrence,biosynthesis and functions[J]. Mini-Rev Med Chem,2017,17 (12):1028−1038.
[25] Ming LG,Lv X,Ma XN,Ge BF,Zhen P,et al. The prenyl group contributes to activities of phytoestrogen 8-prenynaringenin in enhancing bone formation and inhibiting bone resorption in vitro[J]. Endocrinology,2013,154 (3):1202−1214. doi: 10.1210/en.2012-2086
[26] Shi SC,Li JC,Zhao XM,Liu QB,Song SJ. A comprehensive review:biological activity,modification and synthetic methodologies of prenylated flavonoids[J]. Phytochemistry,2021,191:112895. doi: 10.1016/j.phytochem.2021.112895
[27] Yang XM,Jiang YM,Yang JL,He JR,Sun J,et al. Prenylated flavonoids,promising nutraceuticals with impressive biological activities[J]. Trends Food Sci Technol,2015,44 (1):93−104. doi: 10.1016/j.jpgs.2015.03.007
[28] Chen RD,Liu X,Zou JH,Yin YZ,Ou B,et al. Regio- and stereospecific prenylation of flavonoids by Sophora flavescens prenyltransferase[J]. Adv Synth Catal,2013,355 (9):1817−1828. doi: 10.1002/adsc.201300196
[29] Yoneyama K,Akashi T,Aoki T. Molecular characterization of soybean pterocarpan 2-dimethylallyltransferase in glyceollin biosynthesis:local gene and whole-genome duplications of prenyltransferase genes led to the structural diversity of soybean prenylated isoflavonoids[J]. Plant Cell Physiol,2016,57 (12):2497−2509. doi: 10.1093/pcp/pcw178
[30] Akashi T,Sasaki K,Aoki T,Ayabe S,Yazaki K. Molecular cloning and characterization of a cDNA for pterocarpan 4-dimethylallyltransferase catalyzing the key prenylation step in the biosynthesis of glyceollin,a soybean phytoalexin[J]. Plant Physiol,2009,149 (2):683−693. doi: 10.1104/pp.108.123679
[31] Sukumaran A,McDowell T,Chen L,Renaud J,Dhaubhadel S. Isoflavonoid-specific prenyltransferase gene family in soybean:GmPT01,a pterocarpan 2-dimethylallyltransferase involved in glyceollin biosynthesis[J]. Plant J,2018,96 (5):966−981. doi: 10.1111/tpj.14083
[32] Wang RS,Chen RD,Li JH,Liu X,Xie KB,et al. Molecular characterization and phylogenetic analysis of two novel regio-specific flavonoid prenyltransferases from Morus alba and Cudrania tricuspidata[J]. J Biol Chem,2014,289 (52):35815−35825. doi: 10.1074/jbc.M114.608265
[33] Sasaki K,Tsurumaru Y,Yamamoto H,Yazaki K. Molecular characterization of a membrane-bound prenyltransferase specific for isoflavone from Sophora flavescens[J]. J Biol Chem,2011,286 (27):24125−24134. doi: 10.1074/jbc.M111.244426
[34] Shen GA,Huhman D,Lei ZT,Snyder J,Sumner LW,Dixon RA. Characterization of an isoflavonoid-specific prenyltransferase from Lupinus albus[J]. Plant Physiol,2012,159 (1):70−80. doi: 10.1104/pp.112.195271
[35] Li JH,Chen RD,Wang RS,Liu X,Xie KB,et al. Biocatalytic access to diverse prenylflavonoids by combining a regiospecific C-prenyltransferase and a stereospecific chalcone isomerase[J]. Acta Pharm Sin B,2018,8 (4):678−686. doi: 10.1016/j.apsb.2018.01.009
[36] Liu JY,Jiang WB,Xia YY,Wang XM,Shen GA,Pang YZ. Genistein-specific G6DT gene for the inducible production of wighteone in Lotus japonicus[J]. Plant Cell Physiol,2018,59 (1):128−141. doi: 10.1093/pcp/pcx167
[37] He JB,Dong ZY,Hu ZM,Kuang Y,Fan JR,et al. Regio-specific prenylation of pterocarpans by a membrane-bound prenyltransferase from Psoralea corylifolia[J]. Org Biomol Chem,2018,16 (36):6760−6766. doi: 10.1039/C8OB01724G
[38] Rea KA,Casaretto JA,Al-Abdul-Wahid MS,Sukumaran A,Geddes-Mcalister J,et al. Biosynthesis of cannflavins A and B from Cannabis sativa L.[J]. Phytochemistry,2019,164:162−171. doi: 10.1016/j.phytochem.2019.05.009
[39] Tsurumaru Y,Sasaki K,Miyawaki T,Uto Y,Momma T,et al. HlPT-1,a membrane-bound prenyltransferase responsible for the biosynthesis of bitter acids in hops[J]. Biochem Biophys Res Commun,2012,417 (1):393−398. doi: 10.1016/j.bbrc.2011.11.125
[40] Shen G,Luo Y,Yao Y,Meng G,Zhang Y,et al. The discovery of a key prenyltransferase gene assisted by a chromosome-level Epimedium pubescens genome[J]. Front Plant Sci,2022,13:1034943.
[41] Wang PP,Li CJ,Li XD,Huang WJ,Wang Y,et al. Complete biosynthesis of the potential medicine icaritin by engineered Saccharomyces cerevisiae and Escherichia coli[J]. Sci Bull,2021,66 (18):1906−1916. doi: 10.1016/j.scib.2021.03.002
[42] Munakata R,Olry A,Karamat F,Courdavault V,Sugiyama A,et al. Molecular evolution of parsnip (Pastinaca sativa) membrane-bound prenyltransferases for linear and/or angular furanocoumarin biosynthesis[J]. New Phytol,2016,211 (1):332−344. doi: 10.1111/nph.13899
[43] Venugopala KN,Rashmi V,Odhav B. Review on natural coumarin lead compounds for their pharmacological activity[J]. Biomed Res Int,2013,2013:963248.
[44] Karamat F,Olry A,Munakata R,Koeduka T,Sugiyama A,et al. A coumarin-specific prenyltransferase catalyzes the crucial biosynthetic reaction for furanocoumarin formation in parsley[J]. Plant J,2014,77 (4):627−638. doi: 10.1111/tpj.12409
[45] Munakata R,Inoue T,Koeduka T,Karamat F,Olry A,et al. Molecular cloning and characterization of a geranyl diphosphate-specific aromatic prenyltransferase from lemon[J]. Plant Physiol,2014,166 (1):80−90. doi: 10.1104/pp.114.246892
[46] Munakata R,Kitajima S,Nuttens A,Tatsumi K,Takemura T,et al. Convergent evolution of the UbiA prenyltransferase family underlies the independent acquisition of furanocoumarins in plants[J]. New Phytol,2020,225 (5):2166−2182. doi: 10.1111/nph.16277
[47] Munakata R,Olry A,Takemura T,Tatsumi K,Ichino T,et al. Parallel evolution of UbiA superfamily proteins into aromatic O-prenyltransferases in plants[J]. Proc Natl Acad Sci USA,2021,118 (17):e2022294118. doi: 10.1073/pnas.2022294118
[48] Li N,Liu X,Zhang ML,Zhang ZK,Zhang BB,et al. Characterization of a coumarin C-/O-prenyltransferase and a quinolone C-prenyltransferase from Murraya exotica[J]. Org Biomol Chem,2022,20 (28):5535−5542. doi: 10.1039/D2OB01054B
[49] Li HX,Ban ZN,Qin H,Ma LY,King AJ,Wang GD. A heteromeric membrane-bound prenyltransferase complex from hop catalyzes three sequential aromatic prenylations in the bitter acid pathway[J]. Plant Physiol,2015,167 (3):650−659. doi: 10.1104/pp.114.253682
[50] Fiesel T,Gaid M,Müller A,Bartels J,El-Awaad I,et al. Molecular cloning and characterization of a xanthone prenyltransferase from Hypericum calycinum cell cultures[J]. Molecules,2015,20 (9):15616−15630. doi: 10.3390/molecules200915616
[51] Nagia M,Gaid M,Biedermann E,Fiesel T,El-Awaad I,et al. Sequential regiospecific gem-diprenylation of tetrahydroxyxanthone by prenyltransferases from Hypericum sp.[J]. New Phytol,2019,222 (1):318−334. doi: 10.1111/nph.15611
[52] Akinwumi BC,Bordun KAM,Anderson HD. Biological activities of stilbenoids[J]. Int J Mol Sci,2018,19 (3):792. doi: 10.3390/ijms19030792
[53] Yang TH,Fang LL,Sanders S,Jayanthi S,Rajan G,et al. Stilbenoid prenyltransferases define key steps in the diversification of peanut phytoalexins[J]. J Biol Chem,2018,293 (1):28−46. doi: 10.1074/jbc.RA117.000564
[54] Zhong ZH,Zhu W,Liu SZ,Guan QJ,Chen X,et al. Molecular characterization of a geranyl diphosphate-specific prenyltransferase catalyzing stilbenoid prenylation from Morus alba[J]. Plant Cell Physiol,2018,59 (11):2214−2227.
[55] Munakata R,Takemura T,Tatsumi K,Moriyoshi E,Yanagihara K,et al. Isolation of Artemisia capillaris membrane-bound di-prenyltransferase for phenylpropanoids and redesign of artepillin C in yeast[J]. Commun Biol,2019,2:384. doi: 10.1038/s42003-019-0630-0
[56] Saeki H,Hara R,Takahashi H,Iijima M,Munakata R,et al. An aromatic farnesyltransferase functions in biosynthesis of the anti-HIV meroterpenoid daurichromenic acid[J]. Plant Physiol,2018,178 (2):535−551. doi: 10.1104/pp.18.00655
[57] Luo XZ,Reiter MA,D’Espaux L,Wong J,Denby CM,et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast[J]. Nature,2019,567 (7746):123−126. doi: 10.1038/s41586-019-0978-9
[58] Marsafari M,Samizadeh H,Rabiei B,Mehrabi A,Koffas M,Xu P. Biotechnological production of flavonoids:an update on plant metabolic engineering,microbial Host selection,and genetically encoded biosensors[J]. Biotechnol J,2020,15 (8):1900432. doi: 10.1002/biot.201900432
[59] Levisson M,Araya-Cloutier C,de Bruijn WJC,van Der Heide M,Salvador López JM,et al. Toward developing a yeast cell factory for the production of prenylated flavonoids[J]. J Agric Food Chem,2019,67 (49):13478−13486. doi: 10.1021/acs.jafc.9b01367
-
期刊类型引用(0)
其他类型引用(2)
-
其他相关附件
-
DOCX格式
落艳娇附图1-2 点击下载(980KB)
-



 下载:
下载: